An MltA-Like Lytic Transglycosylase Secreted by Bdellovibrio bacteriovorus Cleaves the Prey Septum during Predatory Invasion
- PMID: 37010281
- PMCID: PMC10127604
- DOI: 10.1128/jb.00475-22
An MltA-Like Lytic Transglycosylase Secreted by Bdellovibrio bacteriovorus Cleaves the Prey Septum during Predatory Invasion
Abstract
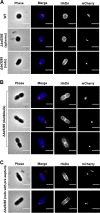
Lytic transglycosylases cut peptidoglycan backbones, facilitating a variety of functions within bacteria, including cell division, pathogenesis, and insertion of macromolecular machinery into the cell envelope. Here, we identify a novel role of a secreted lytic transglycosylase associated with the predatory lifestyle of Bdellovibrio bacteriovorus strain HD100. During wild-type B. bacteriovorus prey invasion, the predator rounds up rod-shaped prey into spherical prey bdelloplasts, forming a spacious niche within which the predator grows. Deleting the MltA-like lytic transglycosylase Bd3285 still permitted predation but resulted in three different, invaded prey cell shapes: spheres, rods, and "dumbbells." Amino acid D321 within the catalytic C-terminal 3D domain of Bd3285 was essential for wild-type complementation. Microscopic analyses revealed that dumbbell-shaped bdelloplasts are derived from Escherichia coli prey undergoing cell division at the moment of Δbd3285 predator invasion. Prelabeling of E. coli prey peptidoglycan prior to predation with the fluorescent D-amino acid HADA showed that the dumbbell bdelloplasts invaded by B. bacteriovorus Δbd3285 contained a septum. Fluorescently tagged Bd3285, expressed in E. coli, localized to the septum of dividing cells. Our data indicate that B. bacteriovorus secretes the lytic transglycosylase Bd3285 into the E. coli periplasm during prey invasion to cleave the septum of dividing prey, facilitating prey cell occupation. IMPORTANCE Antimicrobial resistance is a serious and rapidly growing threat to global health. Bdellovibrio bacteriovorus can prey upon an extensive range of Gram-negative bacterial pathogens and thus has promising potential as a novel antibacterial therapeutic and is a source of antibacterial enzymes. Here, we elucidate the role of a unique secreted lytic transglycosylase from B. bacteriovorus which acts on the septal peptidoglycan of its prey. This improves our understanding of mechanisms that underpin bacterial predation.
Keywords: Bdellovibrio bacteriovorus; MltA; bacterial cell division; bacterial septum; cell wall; lytic transglycosylase; peptidoglycan; peptidoglycan hydrolases; predatory bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
- J Bacteriol. 205.
Similar articles
-
Cleave a Septum, Leave a Cell: Bdellovibrio bacteriovorus Secretes a Specialized Lytic Transglycosylase to Clear Prey Cell Septum Obstruction.J Bacteriol. 2023 Apr 25;205(4):e0007423. doi: 10.1128/jb.00074-23. Epub 2023 Apr 3. J Bacteriol. 2023. PMID: 37010280 Free PMC article.
-
Asymmetric peptidoglycan editing generates cell curvature in Bdellovibrio predatory bacteria.Nat Commun. 2022 Mar 21;13(1):1509. doi: 10.1038/s41467-022-29007-y. Nat Commun. 2022. PMID: 35314810 Free PMC article.
-
Dual Predation by Bacteriophage and Bdellovibrio bacteriovorus Can Eradicate Escherichia coli Prey in Situations where Single Predation Cannot.J Bacteriol. 2020 Feb 25;202(6):e00629-19. doi: 10.1128/JB.00629-19. Print 2020 Feb 25. J Bacteriol. 2020. PMID: 31907203 Free PMC article.
-
Chromosome structure and DNA replication dynamics during the life cycle of the predatory bacterium Bdellovibrio bacteriovorus.FEMS Microbiol Rev. 2023 Nov 1;47(6):fuad057. doi: 10.1093/femsre/fuad057. FEMS Microbiol Rev. 2023. PMID: 37791401 Free PMC article. Review.
-
Bacterial invasion and killing by predatory Bdellovibrio primed by predator prey cell recognition and self protection.Curr Opin Microbiol. 2020 Aug;56:74-80. doi: 10.1016/j.mib.2020.07.002. Epub 2020 Aug 9. Curr Opin Microbiol. 2020. PMID: 32784086 Review.
Cited by
-
Comparative analysis of novel Pseudobdellovibrionaceae genera and species yields insights into the genomics and evolution of bacterial predation mode.bioRxiv [Preprint]. 2025 Feb 20:2025.02.19.638989. doi: 10.1101/2025.02.19.638989. bioRxiv. 2025. PMID: 40027812 Free PMC article. Preprint.
-
Cleave a Septum, Leave a Cell: Bdellovibrio bacteriovorus Secretes a Specialized Lytic Transglycosylase to Clear Prey Cell Septum Obstruction.J Bacteriol. 2023 Apr 25;205(4):e0007423. doi: 10.1128/jb.00074-23. Epub 2023 Apr 3. J Bacteriol. 2023. PMID: 37010280 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

