Laser-Based Mid-Infrared Spectroscopy for Monitoring Temperature-Induced Denaturation of Bovine Serum Albumin and De-/Stabilization Effects of Sugars
- PMID: 37010404
- PMCID: PMC10116488
- DOI: 10.1021/acs.analchem.3c00489
Laser-Based Mid-Infrared Spectroscopy for Monitoring Temperature-Induced Denaturation of Bovine Serum Albumin and De-/Stabilization Effects of Sugars
Abstract
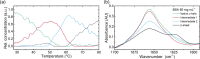
Stability of high-concentration protein formulations is considered a major challenge in current biopharmaceutical development. In this work, we introduce laser-based mid-infrared (IR) spectroscopy as a versatile technique to study the effect of protein concentration and presence of sugars on the thermal denaturation of the model protein bovine serum albumin (BSA). Many analytical techniques struggle to characterize the complex structural transition that occurs during protein denaturation. To this end, a commercially available laser-based mid-IR spectrometer equipped with a customized flow cell was employed to record IR spectra of BSA in the temperature range of 25-85 °C. The temperature perturbation induces a conformational change from a native α-helical to an intermolecular β-sheet secondary structure in BSA. Systematic investigation of the concentration dependence of the α-β transition temperature between 30 and 90 mg mL-1 shows a trend of decreasing denaturation temperatures at higher BSA concentrations. In-depth chemometric analysis by a multivariate curve resolution-alternating least squares (MCR-ALS) analysis of the spectra, suggested the formation of not one but two intermediates in the denaturation of BSA. Subsequently, the impact of sugars on denaturation temperatures was investigated, revealing both stabilizing (trehalose, sucrose, and mannose) and destabilizing (sucralose) effects, illustrating the applicability of this method as an investigative tool for stabilizers. These results highlight the potential and versatility of laser-based IR spectroscopy for analysis of protein stability at high concentrations and varying conditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Preexisting Diabetes and Pregnancy: An Endocrine Society and European Society of Endocrinology Joint Clinical Practice Guideline.Eur J Endocrinol. 2025 Jun 30;193(1):G1-G48. doi: 10.1093/ejendo/lvaf116. Eur J Endocrinol. 2025. PMID: 40652450
-
Dynamic spectroscopic and optical characterization and modeling of bovine serum albumin corona during interaction with N-hydroxysulfo-succinimide-covalently functionalized gold nanourchins.Biointerphases. 2024 Sep 1;19(5):051003. doi: 10.1116/6.0003715. Biointerphases. 2024. PMID: 39356180
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
External Cavity Quantum Cascade Laser Vibrational Circular Dichroism Spectroscopy for Fast and Sensitive Analysis of Proteins at Low Concentrations.Anal Chem. 2024 Dec 10;96(49):19363-19369. doi: 10.1021/acs.analchem.4c03498. Epub 2024 Nov 22. Anal Chem. 2024. PMID: 39576261 Free PMC article.
-
Integrated Optics Waveguides and Mesoporous Oxides for the Monitoring of Volatile Organic Compound Traces in the Mid-Infrared.Appl Spectrosc. 2025 May;79(5):842-851. doi: 10.1177/00037028241300554. Epub 2024 Dec 18. Appl Spectrosc. 2025. PMID: 39692077 Free PMC article.
-
Innovative Infrared Spectroscopic Technologies for the Prediction of Deoxynivalenol in Wheat.ACS Food Sci Technol. 2025 Jan 8;5(1):209-217. doi: 10.1021/acsfoodscitech.4c00730. eCollection 2025 Jan 17. ACS Food Sci Technol. 2025. PMID: 39840402 Free PMC article.
-
Ratiometric luminescent sensor based on BSA-coated gold/silver nanoclusters for the selective determination and spatiotemporal imaging of gallic acid in plants.Mikrochim Acta. 2023 Dec 28;191(1):60. doi: 10.1007/s00604-023-06156-5. Mikrochim Acta. 2023. PMID: 38153646
-
Microbubble-Templated Immunoactive Metal-Phenolic Capsules for Drug Delivery and Enhanced Cancer Immunotherapy.Research (Wash D C). 2025 Jul 4;8:0752. doi: 10.34133/research.0752. eCollection 2025. Research (Wash D C). 2025. PMID: 40625661 Free PMC article.
References
-
- Borzova V. A.; Markossian K. A.; Chebotareva N. A.; Kleymenov S. Yu.; Poliansky N. B.; Muranov K. O.; Stein-Margolina V. A.; Shubin V. V.; Markov D. I.; Kurganov B. I. Kinetics of Thermal Denaturation and Aggregation of Bovine Serum Albumin. PLoS One 2016, 11, e015349510.1371/journal.pone.0153495. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

