Meddling with Metal Sensors: Fur-Family Proteins as Signaling Hubs
- PMID: 37010421
- PMCID: PMC10127796
- DOI: 10.1128/jb.00022-23
Meddling with Metal Sensors: Fur-Family Proteins as Signaling Hubs
Abstract
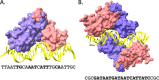
The ferric uptake regulator (Fur) protein is the founding member of the FUR superfamily of metalloregulatory proteins that control metal homeostasis in bacteria. FUR proteins regulate metal homeostasis in response to the binding of iron (Fur), zinc (Zur), manganese (Mur), or nickel (Nur). FUR family proteins are generally dimers in solution, but the DNA-bound complex can involve a single dimer, a dimer-of-dimers, or an extended array of bound protein. Elevated FUR levels due to changes in cell physiology increase DNA occupancy and may also kinetically facilitate protein dissociation. Interactions between FUR proteins and other regulators are commonplace, often including cooperative and competitive DNA-binding interactions within the regulatory region. Further, there are many emerging examples of allosteric regulators that interact directly with FUR family proteins. Here, we focus on newly uncovered examples of allosteric regulation by diverse Fur antagonists (Escherichia coli YdiV/SlyD, Salmonella enterica EIIANtr, Vibrio parahaemolyticus FcrX, Acinetobacter baumannii BlsA, Bacillus subtilis YlaN, and Pseudomonas aeruginosa PacT) as well as one Zur antagonist (Mycobacterium bovis CmtR). Small molecules and metal complexes may also serve as regulatory ligands, with examples including heme binding to Bradyrhizobium japonicum Irr and 2-oxoglutarate binding to Anabaena FurA. How these protein-protein and protein-ligand interactions act in conjunction with regulatory metal ions to facilitate signal integration is an active area of investigation.
Keywords: activator; allosteric regulation; metal homeostasis; metalloregulation; repressor; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

