Depolarization-induced bursts of miniature synaptic currents in individual synapses of developing cerebellum
- PMID: 37010482
- PMCID: PMC10072220
- DOI: 10.1085/jgp.202213212
Depolarization-induced bursts of miniature synaptic currents in individual synapses of developing cerebellum
Abstract
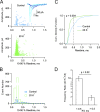
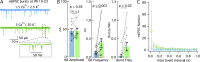
In central synapses, spontaneous transmitter release observed in the absence of action potential firing is often considered as a random process lacking time or space specificity. However, when studying miniature glutamatergic currents at cerebellar synapses between parallel fibers and molecular layer interneurons, we found that these currents were sometimes organized in bursts of events occurring at high frequency (about 30 Hz). Bursts displayed homogeneous quantal size amplitudes. Furthermore, in the presence of the desensitization inhibitor cyclothiazide, successive events within a burst displayed quantal amplitude occlusion. Based on these findings, we conclude that bursts originate in individual synapses. Bursts were enhanced by increasing either the external potassium concentration or the external calcium concentration, and they were strongly inhibited when blocking voltage-gated calcium channels by cadmium. Bursts were prevalent in elevated potassium concentration during the formation of the molecular layer but were infrequent later in development. Since postsynaptic AMPA receptors are largely calcium permeant in developing parallel fiber-interneuron synapses, we propose that bursts involve presynaptic calcium transients implicating presynaptic voltage-gated calcium channels, together with postsynaptic calcium transients implicating postsynaptic AMPA receptors. These simultaneous pre- and postsynaptic calcium transients may contribute to the formation and/or stabilization of synaptic connections.
© 2023 Le Guellec et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

