Outcomes After Endovascular Therapy With Procedural Sedation vs General Anesthesia in Patients With Acute Ischemic Stroke: The AMETIS Randomized Clinical Trial
- PMID: 37010829
- PMCID: PMC10071397
- DOI: 10.1001/jamaneurol.2023.0413
Outcomes After Endovascular Therapy With Procedural Sedation vs General Anesthesia in Patients With Acute Ischemic Stroke: The AMETIS Randomized Clinical Trial
Abstract
Importance: General anesthesia and procedural sedation are common practice for mechanical thrombectomy in acute ischemic stroke. However, risks and benefits of each strategy are unclear.
Objective: To determine whether general anesthesia or procedural sedation for anterior circulation large-vessel occlusion acute ischemic stroke thrombectomy are associated with a difference in periprocedural complications and 3-month functional outcome.
Design, setting, and participants: This open-label, blinded end point randomized clinical trial was conducted between August 2017 and February 2020, with final follow-up in May 2020, at 10 centers in France. Adults with occlusion of the intracranial internal carotid artery and/or the proximal middle cerebral artery treated with thrombectomy were enrolled.
Interventions: Patients were assigned to receive general anesthesia with tracheal intubation (n = 135) or procedural sedation (n = 138).
Main outcomes and measures: The prespecified primary composite outcome was functional independence (a score of 0 to 2 on the modified Rankin Scale, which ranges from 0 [no neurologic disability] to 6 [death]) at 90 days and absence of major periprocedural complications (procedure-related serious adverse events, pneumonia, myocardial infarction, cardiogenic acute pulmonary edema, or malignant stroke) at 7 days.
Results: Among 273 patients evaluable for the primary outcome in the modified intention-to-treat population, 142 (52.0%) were women, and the mean (SD) age was 71.6 (13.8) years. The primary outcome occurred in 38 of 135 patients (28.2%) assigned to general anesthesia and in 50 of 138 patients (36.2%) assigned to procedural sedation (absolute difference, 8.1 percentage points; 95% CI, -2.3 to 19.1; P = .15). At 90 days, the rate of patients achieving functional independence was 33.3% (45 of 135) with general anesthesia and 39.1% (54 of 138) with procedural sedation (relative risk, 1.18; 95% CI, 0.86-1.61; P = .32). The rate of patients without major periprocedural complications at 7 days was 65.9% (89 of 135) with general anesthesia and 67.4% (93 of 138) with procedural sedation (relative risk, 1.02; 95% CI, 0.86-1.21; P = .80).
Conclusions and relevance: In patients treated with mechanical thrombectomy for anterior circulation acute ischemic stroke, general anesthesia and procedural sedation were associated with similar rates of functional independence and major periprocedural complications.
Trial registration: ClinicalTrials.gov Identifier: NCT03229148.
Conflict of interest statement
Figures
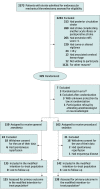


References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 - DOI - PubMed
-
- Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)–European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke: endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. doi: 10.1177/2396987319832140 - DOI - PMC - PubMed
-
- Talke PO, Sharma D, Heyer EJ, Bergese SD, Blackham KA, Stevens RD. Republished: Society for Neuroscience in Anesthesiology and Critical Care expert consensus statement: anesthetic management of endovascular treatment for acute ischemic stroke. Stroke. 2014;45(8):e138-e150. doi: 10.1161/STROKEAHA.113.003412 - DOI - PubMed

