Paracrine relationship between incretin hormones and endogenous 5-hydroxytryptamine in the small and large intestine
- PMID: 37010838
- PMCID: PMC10909488
- DOI: 10.1111/nmo.14589
Paracrine relationship between incretin hormones and endogenous 5-hydroxytryptamine in the small and large intestine
Abstract
Background: Enterochromaffin (EC) cell-derived 5-hydroxytryptamine (5-HT) is a mediator of toxin-induced reflexes, initiating emesis via vagal and central 5-HT3 receptors. The amine is also involved in gastrointestinal (GI) reflexes that are prosecretory and promotile, and recently 5-HT's roles in chemosensation in the distal bowel have been described. We set out to establish the efficacy of 5-HT signaling, local 5-HT levels and pharmacology in discrete regions of the mouse small and large intestine. We also investigated the inter-relationships between incretin hormones, glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) and endogenous 5-HT in mucosal and motility assays.
Methods: Adult mouse GI mucosae were mounted in Ussing chambers and area-specific studies were performed to establish the 5-HT3 and 5-HT4 pharmacology, the sidedness of responses, and the inter-relationships between incretins and endogenous 5-HT. Natural fecal pellet transit in vitro and full-length GI transit in vivo were also measured.
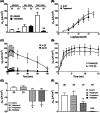
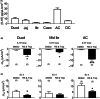
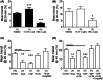
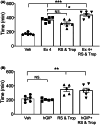
Key results: We observed the greatest level of tonic and exogenous 5-HT-induced ion transport and highest levels of 5-HT in ascending colon mucosa. Here both 5-HT3 and 5-HT4 receptors were involved but elsewhere in the GI tract epithelial basolateral 5-HT4 receptors mediate 5-HT's prosecretory effect. Exendin-4 and GIP induced 5-HT release in the ascending colon, while L cell-derived PYY also contributed to GIP mucosal effects in the descending colon. Both peptides slowed colonic transit.
Conclusions & inferences: We provide functional evidence for paracrine interplay between 5-HT, GLP-1 and GIP, particularly in the colonic mucosal region. Basolateral epithelial 5-HT4 receptors mediated both 5-HT and incretin mucosal responses in healthy colon.
Keywords: 5-hydroxytryptamine; enterochromaffin cells; gastric inhibitory polypeptide; glucagon-like peptide-1; motility; mucosal ion transport.
© 2023 The Authors. Neurogastroenterology & Motility published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Sundler F, Ekblad E, Hakanson R. Localisation and colocalization of gastrointestinal peptides. Handb Exp Pharmacol. 1993;106:1‐28.
-
- Koo A, Fothergill LJ, Kuramoto H, Furness JB. 5‐HT containing enteroendocrine cells characterised by morphologies, patterns of hormone co‐expression and relationships with nerve fibres in the mouse gastrointestinal tract. Histochem Cell Biol. 2021;155:623‐636. - PubMed
-
- Gwynne RM, Bornstein JC. Luminal 5‐HT4 receptors – a successful target for prokinetic actions. Neurogastroenterol Motil. 2019;31:e13708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

