Multicenter, prospective, phase II study of maintenance bevacizumab for children and adults with NF2-related schwannomatosis and progressive vestibular schwannoma
- PMID: 37010875
- PMCID: PMC10398799
- DOI: 10.1093/neuonc/noad066
Multicenter, prospective, phase II study of maintenance bevacizumab for children and adults with NF2-related schwannomatosis and progressive vestibular schwannoma
Abstract
Background: Prospective data on maintenance therapy with bevacizumab for persons with NF2-related schwannomatosis (NF2-SWN) is lacking. In this prospective multicenter phase II study, we evaluated the efficacy, safety, and tolerability of bevacizumab for maintenance therapy in children and adults with NF2-SWN and hearing loss due to vestibular schwannomas (VS).
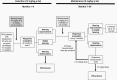
Methods: Following induction therapy, participants received bevacizumab 5 mg/kg every 3 weeks for 18 months. Participants were monitored for changes in hearing, tumor size, and quality of life (QOL), and for adverse events. Hearing loss was defined as a statistically significant decline in word recognition score (WRS) or pure-tone average compared to the study baseline; tumor growth was defined as >20% increase in volume compared to baseline.
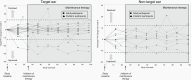
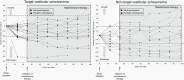
Results: Twenty participants with NF2-SWN (median age 23.5 years; range, 12.5-62.5 years) with hearing loss in the target ear (median WRS 70%, range 2%-94%) received maintenance bevacizumab. Freedom from hearing loss in the target ear was 95% after 48 weeks, 89% after 72 weeks, and 70% after 98 weeks. Freedom from tumor growth in the target VS was 94% after 48 weeks, 89% after 72 weeks, and 89% after 98 weeks. NF2-related QOL remained stable for 98 weeks whereas tinnitus-related distress decreased. Maintenance bevacizumab was well tolerated, with 3 participants (15%) discontinuing treatment due to adverse events.
Conclusions: Maintenance bevacizumab (5 mg/kg every 3 weeks) is associated with high rates of hearing and tumor stability during 18 months of follow-up. No new unexpected adverse events related to bevacizumab were identified in this population.
Keywords: NF2; bevacizumab; maintenance; neurofibromatosis 2; vestibular schwannoma.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
Scott Plotkin: co-founder of NF2 Therapeutics; consultant for Akouos. Jeffrey Allen: No conflicts to report. Girish Dhall: No conflicts to report. Jian Campian: research support from Merck, NeoimmuneTech, Incyte, and GI Innovation. D. Wade Clapp: No conflicts to report. Michael Fisher: Advisory Board for AstraZeneca and Springworks; research support from AstraZeneca, Array Biopharma, and Exelixis. Rakesh Jain: Consultant fees from BMS, Elpis, Innocoll, SPARC, SynDevRx; owns equity in Accurius, Enlight, SynDevRx; Serves on the Board of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, Tekla World Healthcare Fund, and received Research Grants from Boehringer Ingelheim and Sanofi. James Tonsgard: No conflicts to report. Nicole Ullrich: No conflicts to report. Coretta Thomas: No conflicts to report. Lloyd Edwards: No conflicts to report. Bruce Korf: Medical Advisory boards: Genome Medical, Infixion, Healx, and SpringWorks, Recursion. Roger Packer: No conflicts to report. Matthias Karajannis: consultant for CereXis, Recursion. Jaishri Blakeley: Medical Advisory Board for SpringWorks.
Figures


References
-
- Plotkin SR, Messiaen L, Legius E, et al. Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: an international consensus recommendation. Genet Med. 2022;24(9):1967–1977. - PubMed
-
- Plotkin SR, Merker VL, Muzikansky A, et al. Natural history of vestibular schwannoma growth and hearing decline in newly diagnosed neurofibromatosis type 2 patients. Otol Neurotol. 2014;35:e50–e56. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

