Type I Interferon Signaling via the EGR2 Transcriptional Regulator Potentiates CAR T Cell-Intrinsic Dysfunction
- PMID: 37011008
- PMCID: PMC10330003
- DOI: 10.1158/2159-8290.CD-22-1175
Type I Interferon Signaling via the EGR2 Transcriptional Regulator Potentiates CAR T Cell-Intrinsic Dysfunction
Abstract
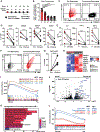
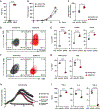
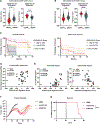
Chimeric antigen receptor (CAR) T cell therapy has shown promise in treating hematologic cancers, but resistance is common and efficacy is limited in solid tumors. We found that CAR T cells autonomously propagate epigenetically programmed type I interferon signaling through chronic stimulation, which hampers antitumor function. EGR2 transcriptional regulator knockout not only blocks this type I interferon-mediated inhibitory program but also independently expands early memory CAR T cells with improved efficacy against liquid and solid tumors. The protective effect of EGR2 deletion in CAR T cells against chronic antigen-induced exhaustion can be overridden by interferon-β exposure, suggesting that EGR2 ablation suppresses dysfunction by inhibiting type I interferon signaling. Finally, a refined EGR2 gene signature is a biomarker for type I interferon-associated CAR T cell failure and shorter patient survival. These findings connect prolonged CAR T cell activation with deleterious immunoinflammatory signaling and point to an EGR2-type I interferon axis as a therapeutically amenable biological system.
Significance: To improve CAR T cell therapy outcomes, modulating molecular determinants of CAR T cell-intrinsic resistance is crucial. Editing the gene encoding the EGR2 transcriptional regulator renders CAR T cells impervious to type I interferon pathway-induced dysfunction and improves memory differentiation, thereby addressing major barriers to progress for this emerging class of cancer immunotherapies. This article is highlighted in the In This Issue feature, p. 1501.
©2023 American Association for Cancer Research.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

