A Compendium of Syngeneic, Transplantable Pediatric High-Grade Glioma Models Reveals Subtype-Specific Therapeutic Vulnerabilities
- PMID: 37011011
- PMCID: PMC10326601
- DOI: 10.1158/2159-8290.CD-23-0004
A Compendium of Syngeneic, Transplantable Pediatric High-Grade Glioma Models Reveals Subtype-Specific Therapeutic Vulnerabilities
Erratum in
-
Correction: A Compendium of Syngeneic, Transplantable Pediatric High-Grade Glioma Models Reveals Subtype-Specific Therapeutic Vulnerabilities.Cancer Discov. 2023 Dec 12;13(12):2674. doi: 10.1158/2159-8290.CD-23-1292. Cancer Discov. 2023. PMID: 38084097 Free PMC article. No abstract available.
-
Correction: A Compendium of Syngeneic, Transplantable Pediatric High-Grade Glioma Models Reveals Subtype-Specific Therapeutic Vulnerabilities.Cancer Discov. 2024 May 1;14(5):891. doi: 10.1158/2159-8290.CD-24-0299. Cancer Discov. 2024. PMID: 38690603 Free PMC article. No abstract available.
Abstract
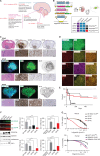
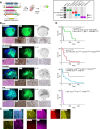
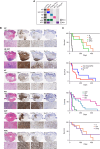
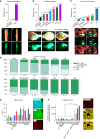
Pediatric high-grade gliomas (pHGG) are lethal, incurable brain tumors frequently driven by clonal mutations in histone genes. They often harbor a range of additional genetic alterations that correlate with different ages, anatomic locations, and tumor subtypes. We developed models representing 16 pHGG subtypes driven by different combinations of alterations targeted to specific brain regions. Tumors developed with varying latencies and cell lines derived from these models engrafted in syngeneic, immunocompetent mice with high penetrance. Targeted drug screening revealed unexpected selective vulnerabilities-H3.3G34R/PDGFRAC235Y to FGFR inhibition, H3.3K27M/PDGFRAWT to PDGFRA inhibition, and H3.3K27M/PDGFRAWT and H3.3K27M/PPM1DΔC/PIK3CAE545K to combined inhibition of MEK and PIK3CA. Moreover, H3.3K27M tumors with PIK3CA, NF1, and FGFR1 mutations were more invasive and harbored distinct additional phenotypes, such as exophytic spread, cranial nerve invasion, and spinal dissemination. Collectively, these models reveal that different partner alterations produce distinct effects on pHGG cellular composition, latency, invasiveness, and treatment sensitivity.
Significance: Histone-mutant pediatric gliomas are a highly heterogeneous tumor entity. Different histone mutations correlate with different ages of onset, survival outcomes, brain regions, and partner alterations. We have developed models of histone-mutant gliomas that reflect this anatomic and genetic heterogeneity and provide evidence of subtype-specific biology and therapeutic targeting. See related commentary by Lubanszky and Hawkins, p. 1516. This article is highlighted in the In This Issue feature, p. 1501.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
-
Modeling the Landscape of Histone-Mutant Pediatric High-Grade Gliomas: A Study in Partner Alterations.Cancer Discov. 2023 Jul 7;13(7):1516-1517. doi: 10.1158/2159-8290.CD-23-0495. Cancer Discov. 2023. PMID: 37416991
References
-
- Ostrom QT, Price M, Ryan K, Edelson J, Neff C, Cioffi G, et al. CBTRUS statistical report: pediatric brain tumor foundation childhood and adolescent primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol 2022;24( Suppl 3):iii1–iii38. - PMC - PubMed
-
- Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012;482:226–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

