Control of stereocilia length during development of hair bundles
- PMID: 37011103
- PMCID: PMC10101650
- DOI: 10.1371/journal.pbio.3001964
Control of stereocilia length during development of hair bundles
Abstract
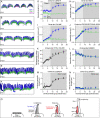
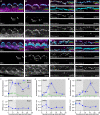
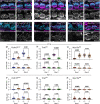
Assembly of the hair bundle, the sensory organelle of the inner ear, depends on differential growth of actin-based stereocilia. Separate rows of stereocilia, labeled 1 through 3 from tallest to shortest, lengthen or shorten during discrete time intervals during development. We used lattice structured illumination microscopy and surface rendering to measure dimensions of stereocilia from mouse apical inner hair cells during early postnatal development; these measurements revealed a sharp transition at postnatal day 8 between stage III (row 1 and 2 widening; row 2 shortening) and stage IV (final row 1 lengthening and widening). Tip proteins that determine row 1 lengthening did not accumulate simultaneously during stages III and IV; while the actin-bundling protein EPS8 peaked at the end of stage III, GNAI3 peaked several days later-in early stage IV-and GPSM2 peaked near the end of stage IV. To establish the contributions of key macromolecular assemblies to bundle structure, we examined mouse mutants that eliminated tip links (Cdh23v2J or Pcdh15av3J), transduction channels (TmieKO), or the row 1 tip complex (Myo15ash2). Cdh23v2J/v2J and Pcdh15av3J/av3J bundles had adjacent stereocilia in the same row that were not matched in length, revealing that a major role of these cadherins is to synchronize lengths of side-by-side stereocilia. Use of the tip-link mutants also allowed us to distinguish the role of transduction from effects of transduction proteins themselves. While levels of GNAI3 and GPSM2, which stimulate stereocilia elongation, were greatly attenuated at the tips of TmieKO/KO row 1 stereocilia, they accumulated normally in Cdh23v2J/v2J and Pcdh15av3J/av3J stereocilia. These results reinforced the suggestion that the transduction proteins themselves facilitate localization of proteins in the row 1 complex. By contrast, EPS8 concentrates at tips of all TmieKO/KO, Cdh23v2J/v2J, and Pcdh15av3J/av3J stereocilia, correlating with the less polarized distribution of stereocilia lengths in these bundles. These latter results indicated that in wild-type hair cells, the transduction complex prevents accumulation of EPS8 at the tips of shorter stereocilia, causing them to shrink (rows 2 and 3) or disappear (row 4 and microvilli). Reduced rhodamine-actin labeling at row 2 stereocilia tips of tip-link and transduction mutants suggests that transduction's role is to destabilize actin filaments there. These results suggest that regulation of stereocilia length occurs through EPS8 and that CDH23 and PCDH15 regulate stereocilia lengthening beyond their role in gating mechanotransduction channels.
Copyright: © 2023 Krey et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures












References
-
- Hudspeth AJ. Hair-bundle mechanics and a model for mechanoelectrical transduction by hair cells. Soc Gen Physiol Ser. 1992;47:357–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

