DR3 Regulates Intestinal Epithelial Homeostasis and Regeneration After Intestinal Barrier Injury
- PMID: 37011811
- PMCID: PMC10213104
- DOI: 10.1016/j.jcmgh.2023.03.008
DR3 Regulates Intestinal Epithelial Homeostasis and Regeneration After Intestinal Barrier Injury
Abstract
Background & aims: Tumor necrosis factor (TNF) superfamily member tumor necrosis factor-like protein 1A (TL1A) has been associated with the susceptibility and severity of inflammatory bowel diseases. However, the function of the tumor necrosis factor-like protein 1A and its receptor death receptor 3 (DR3) in the development of intestinal inflammation is incompletely understood. We investigated the role of DR3 expressed by intestinal epithelial cells (IECs) during intestinal homeostasis, tissue injury, and regeneration.
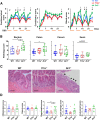
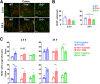
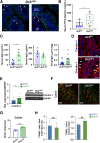
Methods: Clinical phenotype and histologic inflammation were assessed in C57BL/6 (wild-type), Tl1a-/- and Dr3-/- mice in dextran sulfate sodium (DSS)-induced colitis. We generated mice with an IEC-specific deletion of DR3 (Dr3ΔIEC) and assessed intestinal inflammation and epithelial barrier repair. In vivo intestinal permeability was assessed by fluorescein isothiocyanate dextran uptake. Proliferation of IECs was analyzed by bromodeoxyuridine incorporation. Expression of DR3 messenger RNA was assessed by fluorescent in situ hybridization. Small intestinal organoids were used to determine ex vivo regenerative potential.
Results: Dr3-/- mice developed more severe colonic inflammation than wild-type mice in DSS-induced colitis with significantly impaired IEC regeneration. Homeostatic proliferation of IECs was increased in Dr3-/- mice, but blunted during regeneration. Cellular localization and expression of the tight junction proteins Claudin-1 and zonula occludens-1 were altered, leading to increased homeostatic intestinal permeability. Dr3ΔIEC mice recapitulated the phenotype observed in Dr3-/- mice with increased intestinal permeability and IEC proliferation under homeostatic conditions and impaired tissue repair and increased bacterial translocation during DSS-induced colitis. Impaired regenerative potential and altered zonula occludens-1 localization also were observed in Dr3ΔIEC enteroids.
Conclusions: Our findings establish a novel function of DR3 in IEC homeostasis and postinjury regeneration independent of its established role in innate lymphoid cells and T-helper cells.
Keywords: Epithelial Barrier; IEC Proliferation; Intestinal Permeability; Tissue Regeneration.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures











References
-
- Maloy K.J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. - PubMed
-
- Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
-
- Migone T.S., Zhang J., Luo X., et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. - PubMed
-
- Papadakis K.A., Zhu D., Prehn J.L., et al. Dominant role for TL1A/DR3 pathway in IL-12 plus IL-18-induced IFN-gamma production by peripheral blood and mucosal CCR9+ T lymphocytes. J Immunol. 2005;174:4985–4990. - PubMed
-
- Prehn J.L., Thomas L.S., Landers C.J., et al. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007;178:4033–4038. - PubMed

