Off Starburst Amacrine Cells in the Retina Trigger Looming-Evoked Fear Responses in Mice
- PMID: 37011954
- PMCID: PMC10101549
- DOI: 10.1523/ENEURO.0183-22.2023
Off Starburst Amacrine Cells in the Retina Trigger Looming-Evoked Fear Responses in Mice
Abstract
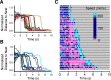
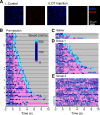
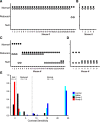
A rapidly approaching dark object evokes an evolutionarily conserved fear response in both vertebrates and invertebrates, young to old. A looming visual stimulus mimics an approaching object and triggers a similarly robust fear response in mice, resulting in freeze and flight. However, the retinal neural pathway responsible for this innate response has not been fully understood. We first explored a variety of visual stimuli that reliably induced these innate responses, and found that a looming stimulus with 2-d acclimation consistently evoked fear responses. Because the fear responses were triggered by the looming stimulus with moving edges, but not by a screen flipping from light to dark, we targeted the starburst amacrine cells (SACs), crucial neurons for retinal motion detection. We used intraocular injection of diphtheria toxin (DT) in mutant mice expressing diphtheria toxin receptors (DTR) in SACs. The looming-evoked fear responses disappeared in half of the DT-injected mice, and the other mice still exhibited the fear responses. The optomotor responses (OMRs) were reduced or eliminated, which occurred independent of the disappearance of the fear responses. A histologic examination revealed that ON SACs were reduced in both mouse groups preserved or absent fear responses. In contrast, the number of OFF SACs was different among two groups. The OFF SACs were relatively preserved in mice exhibiting continued fear responses, whereas they were ablated in mice lacking fear response to looming stimulation. These results indicate that OFF SACs and the direction-selective pathway in the retina play a role in looming-induced fear behaviors.
Keywords: fear response; flight; looming stimulus; starburst amacrine cell; vision test.
Copyright © 2023 Bohl et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
