Pulmonary inflammation promoted by type-2 dendritic cells is a feature of human and murine schistosomiasis
- PMID: 37012228
- PMCID: PMC10070318
- DOI: 10.1038/s41467-023-37502-z
Pulmonary inflammation promoted by type-2 dendritic cells is a feature of human and murine schistosomiasis
Abstract
Schistosomiasis is a parasitic disease affecting over 200 million people in multiple organs, including the lungs. Despite this, there is little understanding of pulmonary immune responses during schistosomiasis. Here, we show type-2 dominated lung immune responses in both patent (egg producing) and pre-patent (larval lung migration) murine Schistosoma mansoni (S. mansoni) infection. Human pre-patent S. mansoni infection pulmonary (sputum) samples revealed a mixed type-1/type-2 inflammatory cytokine profile, whilst a case-control study showed no significant pulmonary cytokine changes in endemic patent infection. However, schistosomiasis induced expansion of pulmonary type-2 conventional dendritic cells (cDC2s) in human and murine hosts, at both infection stages. Further, cDC2s were required for type-2 pulmonary inflammation in murine pre-patent or patent infection. These data elevate our fundamental understanding of pulmonary immune responses during schistosomiasis, which may be important for future vaccine design, as well as for understanding links between schistosomiasis and other lung diseases.
© 2023. The Author(s).
Conflict of interest statement
The Manchester Collaborative Centre for Inflammation Research is a joint venture between the University of Manchester and GSK. The authors working at the MCCIR (A.S.M., E.L.H., A.H.C., S.L.B., A.J.L.R., P.C.C., A.A.G., S.A.C.) declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The remaining authors declare no competing interests.
Figures



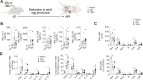


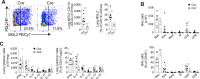
References
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_00033/3/MRC_/Medical Research Council/United Kingdom
- BB/P504543/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/V033417/1/MRC_/Medical Research Council/United Kingdom
- 107475/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- MR/W018748/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

