Intensification of strontium (II) ion biosorption on Sargassum sp via response surface methodology
- PMID: 37012342
- PMCID: PMC10070446
- DOI: 10.1038/s41598-023-32532-5
Intensification of strontium (II) ion biosorption on Sargassum sp via response surface methodology
Abstract
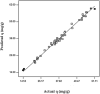
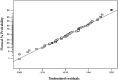
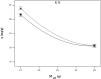
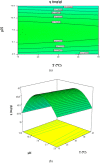
A batch system was employed to investigate the biosorption of strontium (II) on Sargassum sp. The biosorption of strontium on Sargassum sp was studied with response surface methodology to determine the combined effect of temperature, initial metal ion concentration, biomass treatment, biosorbent dosage and pH. Under optimal conditions, the algae's biosorption capacity for strontium (initial pH 7.2, initial strontium concentration 300 mg/l for Mg-treated biomass and biosorbent dosage 0.1 g in 100 mL metal solution) was measured at 103.95 mg/g. In our analysis, equilibrium data were fitted to Langmuir and Freundlich isotherms. Results show that the best fit is provided by the Freundlich model. Biosorption dynamics analysis of the experimental data indicated that strontium (II) was absorbed into algal biomass in accordance with the pseudo-second-order kinetics model well.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ghorbanpour Khamseh AA, Amini Y, Shademan MM, Ghazanfari V. Intensification of thorium biosorption onto protonated orange peel using the response surface methodology. Chem. Prod. Process Model. 2023 doi: 10.1515/cppm-2022-0085. - DOI
-
- Wang L, et al. Adsorption–desorption of strontium from waters using aerobic granules. J. Taiwan Inst. Chem. Eng. 2013;44:454–457. doi: 10.1016/j.jtice.2012.12.005. - DOI
-
- Liu W, et al. Treatment of CrVI-containing Mg (OH)2 Nanowaste. Angew. Chem. 2008;120:5701–5704. doi: 10.1002/ange.200800172. - DOI
-
- Chang Y, et al. Adaptive tracking control for nonlinear system in pure-feedback form with prescribed performance and unknown hysteresis. IMA J. Math. Control. Inf. 2022;39:892–911. doi: 10.1093/imamci/dnac015. - DOI
LinkOut - more resources
Full Text Sources

