LncAABR07053481 inhibits bone marrow mesenchymal stem cell apoptosis and promotes repair following steroid-induced avascular necrosis
- PMID: 37012358
- PMCID: PMC10070412
- DOI: 10.1038/s42003-023-04661-0
LncAABR07053481 inhibits bone marrow mesenchymal stem cell apoptosis and promotes repair following steroid-induced avascular necrosis
Abstract
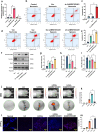
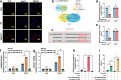
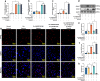
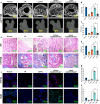
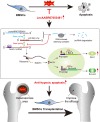
The osteonecrotic area of steroid-induced avascular necrosis of the femoral head (SANFH) is a hypoxic microenvironment that leads to apoptosis of transplanted bone marrow mesenchymal stem cells (BMSCs). However, the underlying mechanism remains unclear. Here, we explore the mechanism of hypoxic-induced apoptosis of BMSCs, and use the mechanism to improve the transplantation efficacy of BMSCs. Our results show that the long non-coding RNA AABR07053481 (LncAABR07053481) is downregulated in BMSCs and closely related to the degree of hypoxia. Overexpression of LncAABR07053481 could increase the survival rate of BMSCs. Further exploration of the downstream target gene indicates that LncAABR07053481 acts as a molecular "sponge" of miR-664-2-5p to relieve the silencing effect of miR-664-2-5p on the target gene Notch1. Importantly, the survival rate of BMSCs overexpressing LncAABR07053481 is significantly improved after transplantation, and the repair effect of BMSCs in the osteonecrotic area is also improved. This study reveal the mechanism by which LncAABR07053481 inhibits hypoxia-induced apoptosis of BMSCs by regulating the miR-664-2-5p/Notch1 pathway and its therapeutic effect on SANFH.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

