Pembrolizumab and cabozantinib in recurrent metastatic head and neck squamous cell carcinoma: a phase 2 trial
- PMID: 37012550
- PMCID: PMC10205145
- DOI: 10.1038/s41591-023-02275-x
Pembrolizumab and cabozantinib in recurrent metastatic head and neck squamous cell carcinoma: a phase 2 trial
Erratum in
-
Author Correction: Pembrolizumab and cabozantinib in recurrent metastatic head and neck squamous cell carcinoma: a phase 2 trial.Nat Med. 2024 Aug;30(8):2373. doi: 10.1038/s41591-024-03161-w. Nat Med. 2024. PMID: 38956200 No abstract available.
Abstract
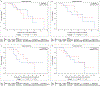
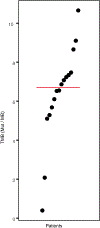
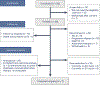
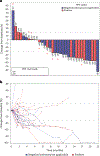
Anti-programmed cell death protein 1 (PD-1) therapy is a standard of care in recurrent metastatic head and neck squamous cell carcinoma (RMHNSCC). Vascular endothelial growth factor inhibitors, including tyrosine kinase inhibitors, have immunomodulatory properties and have offered promising results when combined with anti-PD-1 agents. We conducted a phase 2, multicenter, single-arm trial of pembrolizumab and cabozantinib in patients with RMHNSCC who had Response Evaluation Criteria in Solid Tumors v.1.1 measurable disease and no contraindications to either agent. We assessed the primary end points of tolerability and overall response rate to the combination with secondary end points of progression-free survival and overall survival and performed correlative studies with PDL-1 and combined positive score, CD8+ T cell infiltration and tumor mutational burden. A total of 50 patients were screened and 36 were enrolled with 33 evaluable for response. The primary end point was met, with 17 out of 33 patients having a partial response (52%) and 13 (39%) stable disease with an overall clinical benefit rate of 91%. Median and 1-year overall survival were 22.3 months (95% confidence interval (CI) = 11.7-32.9) and 68.4% (95% CI = 45.1%-83.5%), respectively. Median and 1-year progression-free survival were 14.6 months (95% CI = 8.2-19.6) and 54% (95% CI = 31.5%-72%), respectively. Grade 3 or higher treatment-related adverse events included increased aspartate aminotransferase (n = 2, 5.6%). In 16 patients (44.4%), the dose of cabozantinib was reduced to 20 mg daily. The overall response rate correlated positively with baseline CD8+ T cell infiltration. There was no observed correlation between tumor mutational burden and clinical outcome. Pembrolizumab and cabozantinib were well tolerated and showed promising clinical activity in patients with RMHNSCC. Further investigation of similar combinations are needed in RMHNSCC. The trial is registered at ClinicalTrials.gov under registration no. NCT03468218 .
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
N.F.S. reports advisory roles for Merck, AZ, Eisai, Exelixis, Vaccinex, BNT and CUE. J.E.B. reports advisory roles for Galera Therapeutics and Castle Biosciences. N.C.S. reports research funding from Astex. C.H.C. reports advisory board participation for Merck, Exelixis, Fulgent, Genmab and Brooklyn ImmunoTherapeutics. The other authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

