Dysregulated Ca2+ cycling in atrial fibrillation
- PMID: 37012620
- PMCID: PMC10344643
- DOI: 10.1093/eurheartj/ehad099
Dysregulated Ca2+ cycling in atrial fibrillation
Abstract
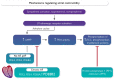
Atrial contractility is regulated by intracellular calcium levels; these reflect the balance of calcium influx, sequestration, and efflux. Beta-adrenergic activation with either norepinephrine (endogenous) or isoproterenol (pharmacological) increases the activity of adenylate cyclase, increasing cAMP levels and protein kinase A (PKA) activity. Degradation of cAMP is regulated by phosphodiesterases (PDEs). The phosphorylation state of relevant PKA targets, such as the L-type calcium channel (LTCC), the sarcoplasmic reticulum Ca2+-ATPase (SERCA2a), and myofilament proteins, is regulated by the balance of PKA activity and phosphatase activity. In patients with no history of atrial fibrillation (AF) or paroxysmal AF (pAF), basal phosphorylation of key LTCC subunits is higher than in atrial myocytes from patients with chronic (persistent) AF. Pavlidou et al. provide novel evidence that this is probably due to increased abundance and activity of phosphodiesterase isoform 8B, variant 2 (PDE8B2), in patients with chronic AF.
Conflict of interest statement
Conflict of interest None declared.
Figures
Comment on
-
Phosphodiesterase 8 governs cAMP/PKA-dependent reduction of L-type calcium current in human atrial fibrillation: a novel arrhythmogenic mechanism.Eur Heart J. 2023 Jul 14;44(27):2483-2494. doi: 10.1093/eurheartj/ehad086. Eur Heart J. 2023. PMID: 36810794 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

