Stress decreases spermatozoa quality and induces molecular alterations in zebrafish progeny
- PMID: 37013516
- PMCID: PMC10071778
- DOI: 10.1186/s12915-023-01570-w
Stress decreases spermatozoa quality and induces molecular alterations in zebrafish progeny
Abstract
Background: Chronic stress can produce a severe negative impact on health not only in the exposed individuals but also in their offspring. Indeed, chronic stress may be contributing to the current worldwide scenario of increasing infertility and decreasing gamete quality in human populations. Here, we evaluate the effect of chronic stress on behavior and male reproductive parameters in zebrafish. Our goal is to provide information on the impact that chronic stress has at molecular, histological, and physiological level in a vertebrate model species.
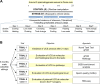
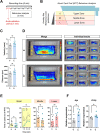
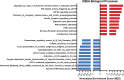
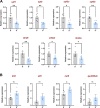
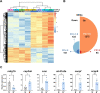
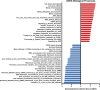
Results: We evaluated the effects of a 21-day chronic stress protocol covering around three full waves of spermatogenesis in Danio rerio adult males. The induction of chronic stress produced anxiety-like behavior in stressed males as assessed by a novel tank test. At a molecular level, the induction of chronic stress consistently resulted in the overexpression of two genes related to endoplasmic reticulum (ER) stress in the brain. Gene set enrichment analysis (GSEA) of testes suggested a dysregulation of the nonsense-mediated decay (NMD) pathway, which was also confirmed on qPCR analysis. Histological analysis of the testicle did not show significant differences in terms of the relative proportions of each germ-cell type; however, the quality of sperm from stressed males was compromised in terms of motility. RNA-seq analysis in stress-derived larval progenies revealed molecular alterations, including those predicted to affect translation initiation, DNA repair, cell cycle control, and response to stress.
Conclusions: Induction of chronic stress during a few cycles of spermatogenesis in the vertebrate zebrafish model affects behavior, gonadal gene expression, final gamete quality, and progeny. The NMD surveillance pathway (a key cellular mechanism that regulates the stability of both normal and mutant transcripts) is severely affected in the testes by chronic stress and therefore the control and regulation of RNAs during spermatogenesis may be affected altering the molecular status in the progeny.
Keywords: Chronic stress; NMD; Progeny; Sperm; Testis; Zebrafish.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- World Health Organization . WHO Fact Sheet: Infertility. 2020.
-
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–1331.e1. doi: 10.1016/j.fertnstert.2012.11.037. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

