Plasmodium falciparum gametocytes display global chromatin remodelling during sexual differentiation
- PMID: 37013531
- PMCID: PMC10071754
- DOI: 10.1186/s12915-023-01568-4
Plasmodium falciparum gametocytes display global chromatin remodelling during sexual differentiation
Abstract
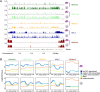
Background: The protozoan malaria parasite Plasmodium falciparum has a complex life cycle during which it needs to differentiate into multiple morphologically distinct life forms. A key process for transmission of the disease is the development of male and female gametocytes in the human blood, yet the mechanisms determining sexual dimorphism in these haploid, genetically identical sexual precursor cells remain largely unknown. To understand the epigenetic program underlying the differentiation of male and female gametocytes, we separated the two sexual forms by flow cytometry and performed RNAseq as well as comprehensive ChIPseq profiling of several histone variants and modifications.
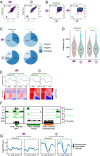
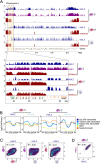
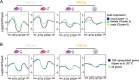
Results: We show that in female gametocytes the chromatin landscape is globally remodelled with respect to genome-wide patterns and combinatorial usage of histone variants and histone modifications. We identified sex specific differences in heterochromatin distribution, implicating exported proteins and ncRNAs in sex determination. Specifically in female gametocytes, the histone variants H2A.Z/H2B.Z were highly enriched in H3K9me3-associated heterochromatin. H3K27ac occupancy correlated with stage-specific gene expression, but in contrast to asexual parasites this was unlinked to H3K4me3 co-occupancy at promoters in female gametocytes.
Conclusions: Collectively, we defined novel combinatorial chromatin states differentially organising the genome in gametocytes and asexual parasites and unravelled fundamental, sex-specific differences in the epigenetic code. Our chromatin maps represent an important resource for future understanding of the mechanisms driving sexual differentiation in P. falciparum.
Keywords: Epigenome; Gametocytogenesis; Histone variants; Malaria; Plasmodium falciparum; Transcriptome.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Heterochromatin dynamics during the initial stages of sexual development in Plasmodium falciparum.Sci Rep. 2024 Oct 5;14(1):23180. doi: 10.1038/s41598-024-73981-w. Sci Rep. 2024. PMID: 39369041 Free PMC article.
-
Comparative Heterochromatin Profiling Reveals Conserved and Unique Epigenome Signatures Linked to Adaptation and Development of Malaria Parasites.Cell Host Microbe. 2018 Mar 14;23(3):407-420.e8. doi: 10.1016/j.chom.2018.01.008. Epub 2018 Mar 1. Cell Host Microbe. 2018. PMID: 29503181 Free PMC article.
-
Expression of P. falciparum var genes involves exchange of the histone variant H2A.Z at the promoter.PLoS Pathog. 2011 Feb;7(2):e1001292. doi: 10.1371/journal.ppat.1001292. Epub 2011 Feb 17. PLoS Pathog. 2011. PMID: 21379342 Free PMC article.
-
Histone globular domain epigenetic modifications: The regulators of chromatin dynamics in malaria parasite.Chembiochem. 2024 Feb 16;25(4):e202300596. doi: 10.1002/cbic.202300596. Epub 2024 Jan 19. Chembiochem. 2024. PMID: 38078518 Review.
-
Chromatin-mediated epigenetic regulation in the malaria parasite Plasmodium falciparum.Eukaryot Cell. 2010 Aug;9(8):1138-49. doi: 10.1128/EC.00036-10. Epub 2010 May 7. Eukaryot Cell. 2010. PMID: 20453074 Free PMC article. Review.
Cited by
-
Heterochromatin dynamics during the initial stages of sexual development in Plasmodium falciparum.Sci Rep. 2024 Oct 5;14(1):23180. doi: 10.1038/s41598-024-73981-w. Sci Rep. 2024. PMID: 39369041 Free PMC article.
-
Sequence-dependent heterochromatin formation in the human malaria parasite Plasmodium falciparum.Heliyon. 2023 Aug 16;9(9):e19164. doi: 10.1016/j.heliyon.2023.e19164. eCollection 2023 Sep. Heliyon. 2023. PMID: 37681121 Free PMC article.
-
PfGCN5 is essential for Plasmodium falciparum survival and transmission and regulates Pf H2B.Z acetylation and chromatin structure.Nucleic Acids Res. 2025 Mar 20;53(6):gkaf218. doi: 10.1093/nar/gkaf218. Nucleic Acids Res. 2025. PMID: 40156869 Free PMC article.
-
MYST regulates DNA repair and forms a NuA4-like complex in the malaria parasite Plasmodium falciparum.mSphere. 2024 Apr 23;9(4):e0014024. doi: 10.1128/msphere.00140-24. Epub 2024 Apr 2. mSphere. 2024. PMID: 38564734 Free PMC article.
-
The exception that proves the rule: Virulence gene expression at the onset of Plasmodium falciparum blood stage infections.PLoS Pathog. 2023 Jun 29;19(6):e1011468. doi: 10.1371/journal.ppat.1011468. eCollection 2023 Jun. PLoS Pathog. 2023. PMID: 37384799 Free PMC article. Clinical Trial.
References
-
- WHO . WHO Malaria Report 2020. 2020.
-
- Lasonder E, Rijpma SR, van Schaijk BC, Hoeijmakers WA, Kensche PR, Gresnigt MS, et al. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. 2016;44:6087–101. doi: 10.1093/nar/gkw536. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

