Liquiritin exhibits anti-acute lung injury activities through suppressing the JNK/Nur77/c-Jun pathway
- PMID: 37013552
- PMCID: PMC10068703
- DOI: 10.1186/s13020-023-00739-3
Liquiritin exhibits anti-acute lung injury activities through suppressing the JNK/Nur77/c-Jun pathway
Abstract
Background: Licorice (Glycyrrhiza uralensis Fisch.), a well-known traditional medicine, is traditionally used for the treatment of respiratory disorders, such as cough, sore throat, asthma and bronchitis. We aim to investigate the effects of liquiritin (LQ), the main bioactive compound in licorice against acute lung injury (ALI) and explore the potential mechanism.
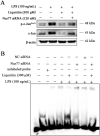
Methods: Lipopolysaccharide (LPS) was used to induce inflammation in RAW264.7 cells and zebrafish. Intratracheal instillation of 3 mg/kg of LPS was used for induction an ALI mice model. The concentrations of IL-6 and TNF-α were tested using the enzyme linked immunosorbent assay. Western blot analysis was used to detect the expression of JNK/Nur77/c-Jun related proteins. Protein levels in bronchoalveolar lavage fluid (BALF) was measured by BCA protein assay. The effect of JNK on Nur77 transcriptional activity was determined by luciferase reporter assay, while electrophoretic mobility shift assay was used to examine the c-Jun DNA binding activity.
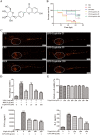
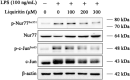
Results: LQ has significant anti-inflammatory effects in zebrafish and RAW264.7 cells. LQ inhibited the expression levels of p-JNK (Thr183/Tyr185), p-Nur77 (Ser351) and p-c-Jun (Ser63), while elevated the Nur77 expression level. Inhibition of JNK by a specific inhibitor or small interfering RNA enhanced the regulatory effect of LQ on Nur77/c-Jun, while JNK agonist abrogated LQ-mediated effects. Moreover, Nur77-luciferase reporter activity was suppressed after JNK overexpression. The effects of LQ on the expression level of c-Jun and the binding activity of c-Jun with DNA were attenuated after Nur77 siRNA treatment. LQ significantly ameliorated LPS-induced ALI with the reduction of lung water content and BALF protein content, the downregulation of TNF-α and IL-6 levels in lung BALF and the suppression of JNK/Nur77/c-Jun signaling, which can be reversed by a specific JNK agonist.
Conclusion: Our results indicated that LQ exerts significant protective effects against LPS-induced inflammation both in vivo and in vitro via suppressing the activation of JNK, and consequently inhibiting the Nur77/c-Jun signaling pathway. Our study suggests that LQ may be a potential therapeutic candidate for ALI and inflammatory disorders.
Keywords: Acute lung injury; JNK; Lipopolysaccharide; Liquiritin; Nur77; c-Jun.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

