Toxic effects of 70% ethanol extract of Moringa stenopetala leaf (Baker f.) Cufod. (Moringaceae) on fetus and placenta of pregnant Wistar rats
- PMID: 37013559
- PMCID: PMC10069107
- DOI: 10.1186/s12906-023-03937-6
Toxic effects of 70% ethanol extract of Moringa stenopetala leaf (Baker f.) Cufod. (Moringaceae) on fetus and placenta of pregnant Wistar rats
Abstract
Background: Moringa stenopetala leaves (Baker f.) Cufod. (Moringaceae) are used as a staple food and traditional medicine for treating various diseases like malaria, hypertension, stomach pain, diabetes, elevated cholesterol, and removing the retained placenta. Its prenatal toxicity study is minimal. Thus, this study aimed to assess the toxic effects of a 70% ethanol extract of Moringa stenopetala leaf on the fetuses and placentas of pregnant Wistar rats.
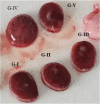
Method: Fresh leaves of Moringa stenopetala were collected, dried at room temperature, ground to powder, and extracted using 70% ethanol. For this study, five groups of animals, each containing ten pregnant rats, were used. Groups I-III were experimental groups and treated with 250, 500, and 1000 mg/kg body weight of Moringa stenopetala leaf extract, respectively. Groups IV and V were pair-fed and ad libitum control groups. The extract was given during gestation days 6 to 12. The fetuses were recovered at day 20 of gestation and examined for the presence of developmental delays, gross external malformations, skeletal and visceral defects. Gross and histopathological changes in the placenta were also evaluated.
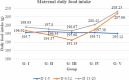
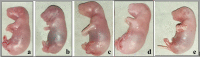
Results: Compared to the pair-fed control group, maternal daily food intake and weight gain were reduced in the 1000 mg/kg-treated group during the treatment and post-treatment periods. A significantly higher number of fetal resorptions was also seen in the 1000 mg/kg treatment group. The crown-rump length and fetal and placental weights were all significantly reduced in pregnant rats given 1000 mg/kg. However, there were no visible malformations in the visceral organs as well as external genitalia in all the treatment and control groups. About 40.7% of the fetuses in the 1000 mg/kg treated rats had no proximal hindlimb phalanges. In addition, light microscopic investigations of the placenta in the high-dose treated rats revealed structural changes in the decidual basalis, trophoblastic zone, and labyrinthine zones.
Conclusion: In conclusion, consumption of M. stenopetala leaves at a higher dose may have toxic effects on the development of rat fetuses. At a higher dose, the plant extract increased the number of fetal resorptions, reduced the number of fetuses, decreased the fetal and placental weights, and alter the placental histopathology. Thus, it is recommended to limit the excess feeding of M. stenopetala leaves during gestation.
Keywords: Developmental retardation; Fetus; Moringa stenopetala; Placenta; Rat; Toxic effect.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


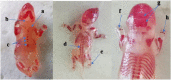
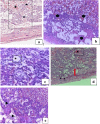
 ); b hemorrhage in the trophoblastic and labyrinth zones (
); b hemorrhage in the trophoblastic and labyrinth zones (
 ); c capillary dilatation (*); d decidual apoptosis (red arrow), decidual cytolysis (black arrows); e decidual necrosis (head arrow); H&E stain, a & b 100× and c, d & e 40× magnification
); c capillary dilatation (*); d decidual apoptosis (red arrow), decidual cytolysis (black arrows); e decidual necrosis (head arrow); H&E stain, a & b 100× and c, d & e 40× magnification
References
-
- Atangwho IJ, et al. Extract of Vernonia amygdalina Del.(African bitter leaf) can reverse pancreatic cellular lesion after alloxan damage in the rat. Aust J Basic Appl Sci. 2010;4(5):711–716.
-
- Cox PA. The ethnobotanical approach to drug discovery: strengths and limitations. Ciba Found Symp. 1994;185:25–36. discussion 36-41. PMID: 7736859. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

