ERK Inhibition Promotes Engraftment of Allografts by Reprogramming T-Cell Metabolism
- PMID: 37013935
- PMCID: PMC10238213
- DOI: 10.1002/advs.202206768
ERK Inhibition Promotes Engraftment of Allografts by Reprogramming T-Cell Metabolism
Abstract
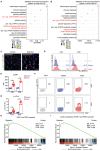
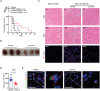
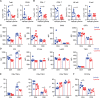
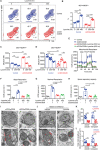
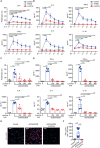
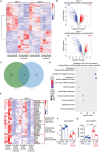
Extracellular regulated protein kinases (ERK) signaling is a master regulator of cell behavior, life, and fate. Although ERK pathway is shown to be involved in T-cell activation, little is known about its role in the development of allograft rejection. Here, it is reported that ERK signaling pathway is activated in allograft-infiltrating T cells. On the basis of surface plasmon resonance technology, lycorine is identified as an ERK-specific inhibitor. ERK inhibition by lycorine significantly prolongs allograft survival in a stringent mouse cardiac allotransplant model. As compared to untreated mice, lycorine-treated mice show a decrease in the number and activation of allograft-infiltrated T cells. It is further confirmed that lycorine-treated mouse and human T cells are less responsive to stimulation in vitro, as indicated by their low proliferative rates and decreased cytokine production. Mechanistic studies reveal that T cells treated with lycorine exhibit mitochondrial dysfunction, resulting in metabolic reprogramming upon stimulation. Transcriptome analysis of lycorine-treated T cells reveals an enrichment in a series of downregulated terms related to immune response, the mitogen-activated protein kinase cascade, and metabolic processes. These findings offer new insights into the development of immunosuppressive agents by targeting the ERK pathway involved in T-cell activation and allograft rejection.
Keywords: allograft rejection; extracellular regulated protein kinases (ERK); lycorine; metabolism; mitochondria.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- a) Bach J. F., Immunol. Today 1993, 14, 322; - PubMed
- b) Burt R. K., Traynor A. E., Pope R., Schroeder J., Cohen B., Karlin K. H., Lobeck L., Goolsby C., Rowlings P., Davis F. A., Stefoski D., Terry C., Keever‐Taylor C., Rosen S., Vesole D., Fishman M., Brush M., Mujias S., Villa M., Burns W. H., Blood 1998, 92, 3505; - PubMed
- c) Sayegh M. H., Carpenter C. B., N. Engl. J. Med. 2004, 351, 2761. - PubMed
-
- Lim M. A., Kohli J., Bloom R. D., Transplant. Rev. (Orlando) 2017, 31, 10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
