Effectors and effects of arginine methylation
- PMID: 37013969
- PMCID: PMC10212539
- DOI: 10.1042/BST20221147
Effectors and effects of arginine methylation
Abstract
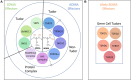
Arginine methylation is a ubiquitous and relatively stable post-translational modification (PTM) that occurs in three types: monomethylarginine (MMA), asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). Methylarginine marks are catalyzed by members of the protein arginine methyltransferases (PRMTs) family of enzymes. Substrates for arginine methylation are found in most cellular compartments, with RNA-binding proteins forming the majority of PRMT targets. Arginine methylation often occurs in intrinsically disordered regions of proteins, which impacts biological processes like protein-protein interactions and phase separation, to modulate gene transcription, mRNA splicing and signal transduction. With regards to protein-protein interactions, the major 'readers' of methylarginine marks are Tudor domain-containing proteins, although additional domain types and unique protein folds have also recently been identified as methylarginine readers. Here, we will assess the current 'state-of-the-art' in the arginine methylation reader field. We will focus on the biological functions of the Tudor domain-containing methylarginine readers and address other domains and complexes that sense methylarginine marks.
Keywords: PRMTs; R-loops; Tudor domains; phase separation.
© 2023 The Author(s).
Conflict of interest statement
M.T.B. is the co-founder of EpiCypher.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

