Prefrontal PV interneurons facilitate attention and are linked to attentional dysfunction in a mouse model of absence epilepsy
- PMID: 37014118
- PMCID: PMC10072875
- DOI: 10.7554/eLife.78349
Prefrontal PV interneurons facilitate attention and are linked to attentional dysfunction in a mouse model of absence epilepsy
Abstract
Absence seizures are characterized by brief periods of unconsciousness accompanied by lapses in motor function that can occur hundreds of times throughout the day. Outside of these frequent moments of unconsciousness, approximately a third of people living with the disorder experience treatment-resistant attention impairments. Convergent evidence suggests prefrontal cortex (PFC) dysfunction may underlie attention impairments in affected patients. To examine this, we use a combination of slice physiology, fiber photometry, electrocorticography (ECoG), optogenetics, and behavior in the Scn8a+/-mouse model of absence epilepsy. Attention function was measured using a novel visual attention task where a light cue that varied in duration predicted the location of a food reward. In Scn8a+/-mice, we find altered parvalbumin interneuron (PVIN) output in the medial PFC (mPFC) in vitro and PVIN hypoactivity along with reductions in gamma power during cue presentation in vivo. This was associated with poorer attention performance in Scn8a+/-mice that could be rescued by gamma-frequency optogenetic stimulation of PVINs. This highlights cue-related PVIN activity as an important mechanism for attention and suggests PVINs may represent a therapeutic target for cognitive comorbidities in absence epilepsy.
Keywords: PV; attention; epilepsy; gamma; interneurons; mouse; neuroscience; prefrontal cortex.
Plain language summary
People who experience absence seizures may go through brief lapses in consciousness hundreds of times a day. They also often have difficulties engaging and remaining focused on a task, which can severely limit their ability to study, work and go through their day-to-day life. These impairments in attention persist even when medication puts a stop to the seizures, suggesting that they are not directly linked to the epileptic episodes. In fact, recent work has indicated that these deficits may be caused instead by alterations in the activity of the prefrontal cortex, the brain area which helps to regulate attention and impulsivity. However, the exact nature of these changes remains unclear, making it difficult to design treatments that could improve patients’ quality of life. To explore this question, Ferguson et al. developed a new behavioral test that allowed them to measure the attention levels of mice genetically engineered to have absence seizures. The experiments confirmed that these animals had impaired attention even when brain activity recordings showed that they were not experiencing seizures. Further work revealed that poor performance on the behavioral test was linked to decreased activity in parvalbumin interneurons, a group of cells in the prefrontal cortex which can inhibit many other types of neurons. In mutant mice, this change was associated with alterations in network activity broadly in the cortex, including in electrical patterns which are linked to cognitive processes. Promisingly, increasing the activity of the interneurons during the attention task improved performance, suggesting that this type of cell could represent a therapeutic target for attention deficit in absence epilepsy.
© 2023, Ferguson et al.
Conflict of interest statement
BF, CG No competing interests declared, JH Reviewing editor, eLife
Figures




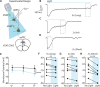





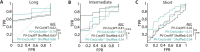




Comment in
-
Attentional Deficits and Absence Epilepsy: A Tale of 2 Interneuronopathies.Epilepsy Curr. 2024 May 13;24(3):188-190. doi: 10.1177/15357597241251709. eCollection 2024 May-Jun. Epilepsy Curr. 2024. PMID: 38898911 Free PMC article. No abstract available.
References
-
- Barker GRI, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. The Journal of Neuroscience. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

