Evolution and immunopathology of chikungunya virus informs therapeutic development
- PMID: 37014125
- PMCID: PMC10110403
- DOI: 10.1242/dmm.049804
Evolution and immunopathology of chikungunya virus informs therapeutic development
Abstract
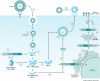
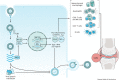
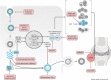
Chikungunya virus (CHIKV), a mosquito-borne alphavirus, is an emerging global threat identified in more than 60 countries across continents. The risk of CHIKV transmission is rising due to increased global interactions, year-round presence of mosquito vectors, and the ability of CHIKV to produce high host viral loads and undergo mutation. Although CHIKV disease is rarely fatal, it can progress to a chronic stage, during which patients experience severe debilitating arthritis that can last from several weeks to months or years. At present, there are no licensed vaccines or antiviral drugs for CHIKV disease, and treatment is primarily symptomatic. This Review provides an overview of CHIKV pathogenesis and explores the available therapeutic options and the most recent advances in novel therapeutic strategies against CHIKV infections.
Keywords: Antiviral compounds; Chikungunya pathogenesis; Chikungunya virus; Immunomodulatory drugs; Monoclonal antibodies; Vaccines.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Ahola, T. and Merits, A. (2016). Functions of chikungunya virus nonstructural proteins. Chikungunya Virus 75-98. 10.1007/978-3-319-42958-8_6 - DOI
-
- Akahata, W., Yang, Z.-Y., Andersen, H., Sun, S., Holdaway, H. A., Kong, W.-P., Lewis, M. G., Higgs, S., Rossmann, M. G., Rao, S.et al. (2010). A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 16, 334-338. 10.1038/nm.2105 - DOI - PMC - PubMed
-
- Bassetto, M., De Burghgraeve, T., Delang, L., Massarotti, A., Coluccia, A., Zonta, N., Gatti, V., Colombano, G., Sorba, G., Silvestri, R.et al. (2013). Computer-aided identification, design and synthesis of a novel series of compounds with selective antiviral activity against chikungunya virus. Antivir. Res. 98, 12-18. 10.1016/j.antiviral.2013.01.002 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

