Molecular characterisation defines clinically-actionable heterogeneity within Group 4 medulloblastoma and improves disease risk-stratification
- PMID: 37014508
- PMCID: PMC10119222
- DOI: 10.1007/s00401-023-02566-0
Molecular characterisation defines clinically-actionable heterogeneity within Group 4 medulloblastoma and improves disease risk-stratification
Abstract
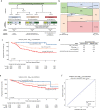
Group 4 tumours (MBGrp4) represent the majority of non-WNT/non-SHH medulloblastomas. Their clinical course is poorly predicted by current risk-factors. MBGrp4 molecular substructures have been identified (e.g. subgroups/cytogenetics/mutations), however their inter-relationships and potential to improve clinical sub-classification and risk-stratification remain undefined. We comprehensively characterised the paediatric MBGrp4 molecular landscape and determined its utility to improve clinical management. A clinically-annotated discovery cohort (n = 362 MBGrp4) was assembled from UK-CCLG institutions and SIOP-UKCCSG-PNET3, HIT-SIOP-PNET4 and PNET HR + 5 clinical trials. Molecular profiling was undertaken, integrating driver mutations, second-generation non-WNT/non-SHH subgroups (1-8) and whole-chromosome aberrations (WCAs). Survival models were derived for patients ≥ 3 years of age who received contemporary multi-modal therapies (n = 323). We first independently derived and validated a favourable-risk WCA group (WCA-FR) characterised by ≥ 2 features from chromosome 7 gain, 8 loss, and 11 loss. Remaining patients were high-risk (WCA-HR). Subgroups 6 and 7 were enriched for WCA-FR (p < 0·0001) and aneuploidy. Subgroup 8 was defined by predominantly balanced genomes with isolated isochromosome 17q (p < 0·0001). While no mutations were associated with outcome and overall mutational burden was low, WCA-HR harboured recurrent chromatin remodelling mutations (p = 0·007). Integration of methylation and WCA groups improved risk-stratification models and outperformed established prognostication schemes. Our MBGrp4 risk-stratification scheme defines: favourable-risk (non-metastatic disease and (i) subgroup 7 or (ii) WCA-FR (21% of patients, 5-year PFS 97%)), very-high-risk (metastatic disease with WCA-HR (36%, 5-year PFS 49%)) and high-risk (remaining patients; 43%, 5-year PFS 67%). These findings validated in an independent MBGrp4 cohort (n = 668). Importantly, our findings demonstrate that previously established disease-wide risk-features (i.e. LCA histology and MYC(N) amplification) have little prognostic relevance in MBGrp4 disease. Novel validated survival models, integrating clinical features, methylation and WCA groups, improve outcome prediction and re-define risk-status for ~ 80% of MBGrp4. Our MBGrp4 favourable-risk group has MBWNT-like excellent outcomes, thereby doubling the proportion of medulloblastoma patients who could benefit from therapy de-escalation approaches, aimed at reducing treatment induced late-effects while sustaining survival outcomes. Novel approaches are urgently required for the very-high-risk patients.
Keywords: Biomarkers; Medulloblastoma; Paediatric Oncology; Risk-stratification.
© 2023. The Author(s).
Conflict of interest statement
S.M.P. reports grants or contracts from ITCC-P4 companies; Lilly, Roche, Pfizer, Charles River, Bayer HealthCare, Pharma Mar, Amgen, Sanofi, Astra Zeneca and Servier. S.Ru. reports participation on advisory boards for Bayer (Germany), Roche (Germany), BMS (Germany) and data safety monitoring board for Cellgene (USA). All other authors declare no competing interests.
Figures



References
-
- Chen Z, Ioris RM, Richardson S, Van Ess AN, Vendrell I, Kessler BM, Buffa FM, Busino L, Clifford SC, Bullock AN, et al (2022) Disease-associated KBTBD4 mutations in medulloblastoma elicit neomorphic ubiquitylation activity to promote CoREST degradation. Cell Death Differ: Doi 10.1038/s41418-022-00983-4 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

