Maternal low-calorie sweetener consumption rewires hypothalamic melanocortin circuits via a gut microbial co-metabolite pathway
- PMID: 37014702
- PMCID: PMC10322686
- DOI: 10.1172/jci.insight.156397
Maternal low-calorie sweetener consumption rewires hypothalamic melanocortin circuits via a gut microbial co-metabolite pathway
Abstract
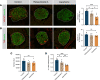
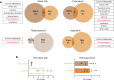
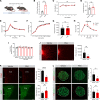
The prevalence of obesity and type 2 diabetes is growing at an alarming rate, including among pregnant women. Low-calorie sweeteners (LCSs) have increasingly been used as an alternative to sugar to deliver a sweet taste without the excessive caloric load. However, there is little evidence regarding their biological effects, particularly during development. Here, we used a mouse model of maternal LCS consumption to explore the impact of perinatal LCS exposure on the development of neural systems involved in metabolic regulation. We report that adult male, but not female, offspring from both aspartame- and rebaudioside A-exposed dams displayed increased adiposity and developed glucose intolerance. Moreover, maternal LCS consumption reorganized hypothalamic melanocortin circuits and disrupted parasympathetic innervation of pancreatic islets in male offspring. We then identified phenylacetylglycine (PAG) as a unique metabolite that was upregulated in the milk of LCS-fed dams and the serum of their pups. Furthermore, maternal PAG treatment recapitulated some of the key metabolic and neurodevelopmental abnormalities associated with maternal LCS consumption. Together, our data indicate that maternal LCS consumption has enduring consequences on the offspring's metabolism and neural development and that these effects are likely to be mediated through the gut microbial co-metabolite PAG.
Keywords: Imprinting; Melanocortin; Metabolism; Neuroendocrine regulation; Neuroscience.
Figures