A seeding-based neuronal model of tau aggregation for use in drug discovery
- PMID: 37014877
- PMCID: PMC10072482
- DOI: 10.1371/journal.pone.0283941
A seeding-based neuronal model of tau aggregation for use in drug discovery
Abstract
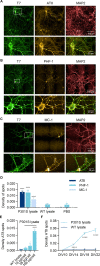
Intracellular accumulation of tau protein is a hallmark of Alzheimer's Disease and Progressive Supranuclear Palsy, as well as other neurodegenerative disorders collectively known as tauopathies. Despite our increasing understanding of the mechanisms leading to the initiation and progression of tau pathology, the field still lacks appropriate disease models to facilitate drug discovery. Here, we established a novel and modulatable seeding-based neuronal model of full-length 4R tau accumulation using humanized mouse cortical neurons and seeds from P301S human tau transgenic animals. The model shows specific and consistent formation of intraneuronal insoluble full-length 4R tau inclusions, which are positive for known markers of tau pathology (AT8, PHF-1, MC-1), and creates seeding competent tau. The formation of new inclusions can be prevented by treatment with tau siRNA, providing a robust internal control for use in qualifying the assessment of potential therapeutic candidates aimed at reducing the intracellular pool of tau. In addition, the experimental set up and data analysis techniques used provide consistent results in larger-scale designs that required multiple rounds of independent experiments, making this is a versatile and valuable cellular model for fundamental and early pre-clinical research of tau-targeted therapies.
Copyright: © 2023 Amorim et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

