ITK degradation to block T cell receptor signaling and overcome therapeutic resistance in T cell lymphomas
- PMID: 37015223
- PMCID: PMC10151063
- DOI: 10.1016/j.chembiol.2023.03.007
ITK degradation to block T cell receptor signaling and overcome therapeutic resistance in T cell lymphomas
Abstract
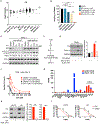
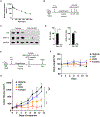
Interleukin (IL)-2-inducible T cell kinase (ITK) is essential for T cell receptor (TCR) signaling and plays an integral role in T cell proliferation and differentiation. Unlike the ITK homolog BTK, no inhibitors of ITK are currently US Food and Drug Administration (FDA) approved. In addition, recent studies have identified mutations within BTK that confer resistance to both covalent and non-covalent inhibitors. Here, as an alternative strategy, we report the development of BSJ-05-037, a potent and selective heterobifunctional degrader of ITK. BSJ-05-037 displayed enhanced anti-proliferative effects relative to its parent inhibitor BMS-509744, blocked the activation of NF-kB/GATA-3 signaling, and increased the sensitivity of T cell lymphoma cells to cytotoxic chemotherapy both in vitro and in vivo. In summary, targeted degradation of ITK is a novel approach to modulate TCR signal strength that could have broad application for the investigation and treatment of T cell-mediated diseases.
Keywords: GATA-3; ITK; PROTAC; T cell lymphoma; TCR signaling.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests N.S.G., D.M.W., B.S.J., and W.W. are inventors on a patent application related to the ITK degrader described in this manuscript. N.S.G. is a founder, scientific advisory board (SAB) member, and equity holder in Syros, C4, Allorion, Lighthorse, Voronoi, Inception, Matchpoint, CobroVentures, GSK, Shenandoah (board member), Larkspur (board member), and Soltego (board member). The Gray lab receives or has received research funding from Novartis, Takeda, Astellas, Taiho, Jansen, Kinogen, Arbella, Deerfield, Springworks, Interline, and Sanofi. D.M.W. is an employee of Merck and a founder, SAB member, and equity holder in Ajax and Travera. The Weinstock lab received research funding from AstraZeneca, Daiichi Sankyo, Secura, and Abcuro. E.S.F. is a founder, member of the SAB, and equity holder of Civetta Therapeutics, Jengu Therapeutics, Proximity Therapeutics, and Neomorph Inc.; SAB member and equity holder in Avilar Therapeutics and Photys Therapeutics; and a consultant to Astellas, Sanofi, Novartis, Deerfield, and EcoR1 capital. The Fischer laboratory receives or has received research funding from Novartis, Deerfield, Ajax, Interline, and Astellas. K.A.D. is a consultant to Kronos Bio and Neomorph Inc.
Figures


Comment in
-
Degrading the signal amplifier: ITK as a target for targeted protein degradation.Cell Chem Biol. 2023 Apr 20;30(4):337-339. doi: 10.1016/j.chembiol.2023.04.001. Cell Chem Biol. 2023. PMID: 37084715
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

