Free-Breathing Liver Fat, R₂* and B₀ Field Mapping Using Multi-Echo Radial FLASH and Regularized Model-Based Reconstruction
- PMID: 37015368
- PMCID: PMC10368089
- DOI: 10.1109/TMI.2022.3228075
Free-Breathing Liver Fat, R₂* and B₀ Field Mapping Using Multi-Echo Radial FLASH and Regularized Model-Based Reconstruction
Abstract
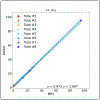
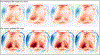
This work introduced a stack-of-radial multi-echo asymmetric-echo MRI sequence for free-breathing liver volumetric acquisition. Regularized model-based reconstruction was implemented in Berkeley Advanced Reconstruction Toolbox (BART) to jointly estimate all physical parameter maps (water, fat, R2∗ , and B0 field inhomogeneity maps) and coil sensitivity maps from self-gated k -space data. Specifically, locally low rank and temporal total variation regularization were employed directly on physical parameter maps. The proposed free-breathing radial technique was tested on a water/fat & iron phantom, a young volunteer, and obesity/diabetes/hepatic steatosis patients. Quantitative fat fraction and R2∗ accuracy were confirmed by comparing our technique with the reference breath-hold Cartesian scan. The multi-echo radial sampling sequence achieves fast k -space coverage and is robust to motion. Moreover, the proposed motion-resolved model-based reconstruction allows for free-breathing liver fat and R2∗ quantification in multiple motion states. Overall, our proposed technique offers a convenient tool for non-invasive liver assessment with no breath holding requirement.
Figures









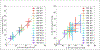

References
-
- Hu HH, Branca RT, Hernando D, Karampinos DC, Machann J, McKenzie CA, Wu HH, Yokoo T, and Velan SS, “Magnetic resonance imaging of obesity and metabolic disorders: Summary from the 2019 ISMRM Workshop,” Magn. Reson. Med, vol. 83, pp. 1565–1576, 2020. - PubMed
-
- Wood JC, “Impact of iron assessment by MRI,” Hematology, vol. 2011, pp. 443–450, 2011. - PubMed
-
- Dixon WT, “Simple proton spectroscopic imaging,” Radiology, vol. 153, pp. 189–194, 1984. - PubMed

