Interleukin (IL)-1 Receptor Signaling Is Required for Complete Taste Bud Regeneration and the Recovery of Neural Taste Responses following Axotomy
- PMID: 37015809
- PMCID: PMC10184746
- DOI: 10.1523/JNEUROSCI.1355-22.2023
Interleukin (IL)-1 Receptor Signaling Is Required for Complete Taste Bud Regeneration and the Recovery of Neural Taste Responses following Axotomy
Abstract
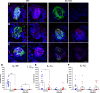
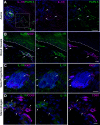
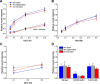
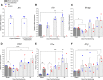
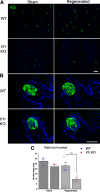
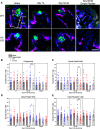
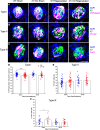
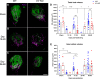
Experimental or traumatic nerve injury causes the degeneration of associated taste buds. Unlike most sensory systems, the sectioned nerve and associated taste buds can then regenerate, restoring neural responses to tastants. It was previously unknown whether injury-induced immune factors mediate this process. The proinflammatory cytokines, interleukin (IL)-1α and IL-1β, and their requisite receptor are strongly expressed by anterior taste buds innervated by the chorda tympani nerve. We tested taste bud regeneration and functional recovery in mice lacking the IL-1 receptor. After axotomy, the chorda tympani nerve regenerated but was initially unresponsive to tastants in both WT and Il1r KO mice. In the absence of Il1r signaling, however, neural taste responses remained minimal even >8 weeks after injury in both male and female mice, whereas normal taste function recovered by 3 weeks in WT mice. Failed recovery was because of a 57.8% decrease in regenerated taste buds in Il1r KO compared with WT axotomized mice. Il1a gene expression was chronically dysregulated, and the subset of regenerated taste buds were reinnervated more slowly and never reached full volume as progenitor cell proliferation lagged in KO mice. Il1r signaling is thus required for complete taste bud regeneration and the recovery of normal taste transmission, likely by impairing taste progenitor cell proliferation. This is the first identification of a cytokine response that promotes taste recovery. The remarkable plasticity of the taste system makes it ideal for identifying injury-induced mechanisms mediating successful regeneration and recovery.SIGNIFICANCE STATEMENT Taste plays a critical role in nutrition and quality of life. The adult taste system is highly plastic and able to regenerate following the disappearance of most taste buds after experimental nerve injury. Several growth factors needed for taste bud regeneration have been identified, but we demonstrate the first cytokine pathway required for the recovery of taste function. In the absence of IL-1 cytokine signaling, taste bud regeneration is incomplete, preventing the transmission of taste activity to the brain. These results open a new direction in revealing injury-specific mechanisms that could be harnessed to promote the recovery of taste perception after trauma or disease.
Keywords: chorda tympani nerve; cytokine; degeneration; electrophysiology; inflammation; taste receptor cell.
Copyright © 2023 the authors.
Figures

Similar articles
-
BDNF is required for taste axon regeneration following unilateral chorda tympani nerve section.Exp Neurol. 2017 Jul;293:27-42. doi: 10.1016/j.expneurol.2017.03.016. Epub 2017 Mar 25. Exp Neurol. 2017. PMID: 28347764 Free PMC article.
-
Recovery of chorda tympani nerve function following injury.Exp Neurol. 1996 Oct;141(2):337-46. doi: 10.1006/exnr.1996.0169. Exp Neurol. 1996. PMID: 8812170
-
Observation of regenerated fungiform taste buds after severing the chorda tympani nerve using confocal laser scanning microscopy in vivo.Otol Neurotol. 2014 Mar;35(3):e110-6. doi: 10.1097/MAO.0000000000000223. Otol Neurotol. 2014. PMID: 24492136
-
Response of the gustatory system to peripheral nerve injury.Exp Neurol. 1992 Jan;115(1):60-4. doi: 10.1016/0014-4886(92)90222-c. Exp Neurol. 1992. PMID: 1728574 Review.
-
[Progress in the effects of injury and regeneration of gustatory nerves on the taste functions in animals].Sheng Li Xue Bao. 2014 Oct 25;66(5):519-27. Sheng Li Xue Bao. 2014. PMID: 25331997 Review. Chinese.
Cited by
-
Connection between nutrition and oncology in dogs and cats: perspectives, evidence, and implications-a comprehensive review.Front Vet Sci. 2025 Feb 18;11:1490290. doi: 10.3389/fvets.2024.1490290. eCollection 2024. Front Vet Sci. 2025. PMID: 40046187 Free PMC article. Review.
-
Altered peripheral taste function in a mouse model of inflammatory bowel disease.Sci Rep. 2023 Nov 2;13(1):18895. doi: 10.1038/s41598-023-46244-3. Sci Rep. 2023. PMID: 37919307 Free PMC article.
-
Give-and-take of gustation: the interplay between gustatory neurons and taste buds.Chem Senses. 2024 Jan 1;49:bjae029. doi: 10.1093/chemse/bjae029. Chem Senses. 2024. PMID: 39078723 Free PMC article. Review.
-
Taste cells depend on axon proximity to generate presynaptic sites.PLoS One. 2025 Jun 3;20(6):e0325312. doi: 10.1371/journal.pone.0325312. eCollection 2025. PLoS One. 2025. PMID: 40460158 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
