FHL2 deficiency impairs follicular development and fertility by attenuating EGF/EGFR/YAP signaling in ovarian granulosa cells
- PMID: 37015904
- PMCID: PMC10073124
- DOI: 10.1038/s41419-023-05759-3
FHL2 deficiency impairs follicular development and fertility by attenuating EGF/EGFR/YAP signaling in ovarian granulosa cells
Abstract
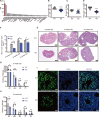
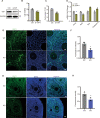
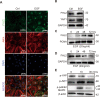
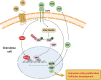
Female subfertility is an increasing reproductive issue worldwide, which is partially related to abnormal ovarian follicular development. Granulosa cells (GCs), by providing the necessary physical support and microenvironment for follicular development, play critical roles in maintaining female fertility. We previously showed that ectopic expression of four and a half LIM domains 2 (FHL2) promoted ovarian granulosa cell tumor progression. However, its function in follicular development and fertility remains unknown. Here, we confirmed that FHL2 is highly expressed in human and mouse ovaries. FHL2 immunosignals were predominantly expressed in ovarian GCs. A Fhl2 knockout (KO) mouse model was generated to examine its roles in follicular development and fertility. Compared with wildtype, knockout of Fhl2 significantly decreased female litter size and offspring number. Furthermore, Fhl2 deficiency reduced ovarian size and impaired follicular development. RNA-sequencing analysis of GCs isolated from either KO or WT mice revealed that, Fhl2 deletion impaired multiple biological functions and signaling pathways, such as Ovarian Putative Early Atresia Granulosa Cell, ErbB, Hippo/YAP, etc. In vitro studies confirmed that FHL2 silencing suppressed GCs growth and EGF-induced GCs proliferation, while its overexpression promoted GC proliferation and decreased apoptosis. Mechanistic studies indicated that FHL2, via forming complexes with transcriptional factors AP-1 or NF-κB, regulated Egf and Egfr expression, respectively. Besides, FHL2 depletion decreased YAP1 expression, especially the active form of YAP1 (nuclear YAP1) in GCs of growing follicles. EGF, serving as an autocrine/paracrine factor, not only induced FHL2 expression and nuclear accumulation, but also stimulated YAP1 expression and activation. Collectively, our study suggests that FHL2 interacts with EGFR and Hippo/YAP signaling to regulate follicular development and maintain fertility. This study illuminates a novel mechanism for follicular development and a potential therapeutic target to address subfertility.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Farquhar CM, Bhattacharya S, Repping S, Mastenbroek S, Kamath MS, Marjoribanks J, et al. Female subfertility. Nat Rev Dis Primers. 2019;5:1–21. - PubMed
-
- Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103:303–16. - PubMed
-
- Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, et al. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

