Factors impacting the efficacy of the in-situ vaccine with CpG and OX40 agonist
- PMID: 37016127
- PMCID: PMC10264285
- DOI: 10.1007/s00262-023-03433-3
Factors impacting the efficacy of the in-situ vaccine with CpG and OX40 agonist
Abstract
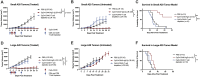
Background: The in-situ vaccine using CpG oligodeoxynucleotide combined with OX40 agonist antibody (CpG + OX40) has been shown to be an effective therapy activating an anti-tumor T cell response in certain settings. The roles of tumor volume, tumor model, and the addition of checkpoint blockade in the efficacy of CpG + OX40 in-situ vaccination remains unknown.
Methods: Mice bearing flank tumors (B78 melanoma or A20 lymphoma) were treated with combinations of CpG, OX40, and anti-CTLA-4. Tumor growth and survival were monitored. In vivo T cell depletion, tumor cell phenotype, and tumor infiltrating lymphocyte (TIL) studies were performed. Tumor cell sensitivity to CpG and macrophages were evaluated in vitro.
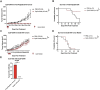
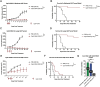
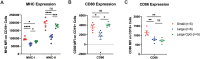
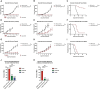
Results: As tumor volumes increased in the B78 (one-tumor) and A20 (one-tumor or two-tumor) models, the anti-tumor efficacy of the in-situ vaccine decreased. In vitro, CpG had a direct effect on A20 proliferation and phenotype and an indirect effect on B78 proliferation via macrophage activation. As A20 tumors progressed in vivo, tumor cell phenotype changed, and T cells became more involved in the local CpG + OX40 mediated anti-tumor response. In mice with larger tumors that were poorly responsive to CpG + OX40, the addition of anti-CTLA-4 enhanced the anti-tumor efficacy in the A20 but not B78 models.
Conclusions: Increased tumor volume negatively impacts the anti-tumor capability of CpG + OX40 in-situ vaccine. The addition of checkpoint blockade augmented the efficacy of CpG + OX40 in the A20 but not B78 model. These results highlight the importance of considering multiple preclinical model conditions when assessing the efficacy of cancer immunotherapy regimens and their translation to clinical testing.
Keywords: Anti-CTLA-4; CpG; In-situ vaccine; OX40 agonist; Preclinical tumor progression.
© 2023. The Author(s).
Conflict of interest statement
ZSM is a member of the scientific advisory board for Archeus Technologies and Seneca Therapeutics and received equity options for these companies. ZSM is an inventor on patents or filed patents managed by the Wisconsin Alumni Research Foundation relating to the interaction of targeted radionuclide therapies and immunotherapies, nanoparticles designed to augment the anti-tumor immune response following radiation therapy, and the development of a brachytherapy catheter capable of delivering intra-tumor injectables. PMS is an inventor on patents or filed patents managed by the Wisconsin Alumni Research Foundation relating to mAb-related immunotherapies and the interaction of targeted radionuclide therapies and immunotherapies.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

