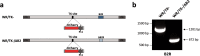
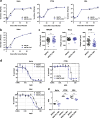
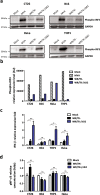
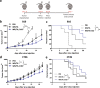
Improving poxvirus-mediated antitumor immune responses by deleting viral cGAMP-specific nuclease
- PMID: 37016144
- PMCID: PMC10353925
- DOI: 10.1038/s41417-023-00610-5
Improving poxvirus-mediated antitumor immune responses by deleting viral cGAMP-specific nuclease
Abstract
cGAMP-specific nucleases (poxins) are a recently described family of proteins dedicated to obstructing cyclic GMP-AMP synthase signaling (cGAS), an important sensor triggered by cytoplasmic viral replication that activates type I interferon (IFN) production. The B2R gene of vaccinia viruses (VACV) codes for one of these nucleases. Here, we evaluated the effects of inactivating the VACV B2 nuclease in the context of an oncolytic VACV. VACV are widely used as anti-cancer vectors due to their capacity to activate immune responses directed against tumor antigens. We aimed to elicit robust antitumor immunity by preventing viral inactivation of the cGAS/STING/IRF3 pathway after infection of cancer cells. Activation of such a pathway is associated with a dominant T helper 1 (Th1) cell differentiation of the response, which benefits antitumor outcomes. Deletion of the B2R gene resulted in enhanced IRF3 phosphorylation and type I IFN expression after infection of tumor cells, while effective VACV replication remained unimpaired, both in vitro and in vivo. In syngeneic mouse tumor models, the absence of the VACV cGAMP-specific nuclease translated into improved antitumor activity, which was associated with antitumor immunity directed against tumor epitopes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures