Real-World Outcomes Following First-Line Treatment in Patients with Advanced Ovarian Cancer with Multiple Risk Factors for Disease Progression who Received Maintenance Therapy or Active Surveillance
- PMID: 37016186
- PMCID: PMC10260707
- DOI: 10.1007/s40487-023-00227-6
Real-World Outcomes Following First-Line Treatment in Patients with Advanced Ovarian Cancer with Multiple Risk Factors for Disease Progression who Received Maintenance Therapy or Active Surveillance
Abstract
Introduction: We evaluated real-world outcomes in patients with advanced ovarian cancer (AOC) based on their cumulative risk profile and maintenance therapy (MT) status following first-line (1L) treatment.
Methods: This retrospective observational study of a nationwide electronic health record-derived de-identified database included adult patients diagnosed with stage III/IV OC from January 1, 2011 to February 28, 2021, who received 1L therapy and had ≥ 12 weeks of follow-up after the index date (end of 1L therapy). Patients were grouped according to whether they received MT or active surveillance (AS) following 1L treatment and by the cumulative number of risk factors (RF) present (stage IV disease; no surgery/treated with neoadjuvant therapy and interval debulking surgery; had postoperative visible residual disease; and had BRCA wild-type disease/unknown BRCA status). Time to next treatment (TTNT) and overall survival (OS) were assessed with a cloning and inverse probability of censoring (IPC)-weighted Kaplan-Meier method.
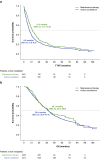
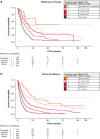
Results: Among 1920 patients, 22.2% received MT and 77.8% received AS. Median IPC-weighted TTNT and OS were 13.3 months (95% CI 11.7-15.8) and 39.1 months (95% CI 32.5-48.6) in the MT cohort, respectively, and 8.6 months (95% CI 8.0-9.5) and 38.4 months (95% CI 36.4-41.0) in the AS cohort, respectively. Almost all patients had ≥ 1 RF (MT 95.3%; AS 96.7%). Median IPC-weighted TTNT was shorter among patients with more RF in both cohorts (MT: 1 RF, 19.3 months, 95% CI 13.5-37.8; 2 RF, 17.2 months, 95% CI 12.8-20.2; 3 RF, 11.0 months, 95% CI 8.2-13.8; 4 RF, 7.0 months, 95% CI 6.2-8.8; AS: 1 RF, 17.7 months, 95% CI 13.5-22.3; 2 RF, 10.2 months, 95% CI 9.1-11.5; 3 RF, 6.5 months, 95% CI 5.8-7.4; 4 RF, 4.1 months, 95% CI 3.5-4.5).
Conclusion: Regardless of RF number, MT was associated with longer TTNT in real-world patients with AOC.
Keywords: Electronic health records; Maintenance therapy; Ovarian cancer; Risk factors.
© 2023. The Author(s).
Conflict of interest statement
Dana Chase reports speakers’ bureau fees and/or advisory roles from, AstraZeneca, Clovis, Genentech/Roche, and GSK and consulting fees from GSK, AstraZeneca, Clovis, and Genentech/Roche. Jessica Perhanidis is a current employee of GSK and reports financial interest (stock) in Boston Scientific and GSK. Linda Kalilani and Amanda Golembesky are current employees of GSK. Divya Gupta was an employee of GSK at the time the analysis was conducted, and is currently affiliated with Mersana Therapeutics, Inc, Cambridge, MA, USA. Antonio González-Martín reports honoraria as advisor from Alkermes, Amgen, AstraZeneca, Clovis Oncology, Genmab, GSK, HederaDx, ImmunoGen, Merck Sharp & Dohme, MacroGenics, Novartis, Oncoinvent, Pfizer/Merck, PharmaMar, Roche, Sotio, Sutro; honoraria as speaker for AstraZeneca, Clovis, GSK, PharmaMar, Roche; institutional funding (GEICO) for clinical research from Roche and GSK; and non-remunerated activities as chair in GEICO and ENGOT (for the period 2018–2020).
Figures



Similar articles
-
Prescribers and patients drive maintenance therapy patterns in a community oncology practice: National guidelines versus the real-world experience.Gynecol Oncol Rep. 2024 Jun 25;54:101440. doi: 10.1016/j.gore.2024.101440. eCollection 2024 Aug. Gynecol Oncol Rep. 2024. PMID: 39040942 Free PMC article.
-
Real-World First-Line Maintenance Niraparib Monotherapy Use Following Chemotherapy Plus Bevacizumab: The SW1TCH Study.Oncol Ther. 2024 Sep;12(3):465-475. doi: 10.1007/s40487-024-00281-8. Epub 2024 Jul 4. Oncol Ther. 2024. PMID: 38965204 Free PMC article.
-
Real-world overall survival in second-line maintenance niraparib monotherapy versus active surveillance in patients with BRCA wild-type recurrent ovarian cancer.Ther Adv Med Oncol. 2024 Nov 14;16:17588359241292272. doi: 10.1177/17588359241292272. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39552638 Free PMC article.
-
Association of Multiple High-Risk Factors on Observed Outcomes in Real-World Patients With Advanced Ovarian Cancer Treated With First-Line Therapy.JCO Clin Cancer Inform. 2023 Jun;7:e2200189. doi: 10.1200/CCI.22.00189. JCO Clin Cancer Inform. 2023. PMID: 37294913 Free PMC article.
-
Niraparib as First-Line Maintenance Therapy in Patients with Stage III Ovarian Cancer and No Visible Residual Disease: AR1ZE Real-World Study.Oncol Ther. 2025 Mar;13(1):253-262. doi: 10.1007/s40487-024-00318-y. Epub 2025 Jan 7. Oncol Ther. 2025. PMID: 39776033 Free PMC article.
Cited by
-
Prescribers and patients drive maintenance therapy patterns in a community oncology practice: National guidelines versus the real-world experience.Gynecol Oncol Rep. 2024 Jun 25;54:101440. doi: 10.1016/j.gore.2024.101440. eCollection 2024 Aug. Gynecol Oncol Rep. 2024. PMID: 39040942 Free PMC article.
-
Patient Characteristics Associated with Time to Next Treatment in Patients with Ovarian Cancer Treated with Niraparib: The PRED1CT Real-World Study.Oncol Ther. 2024 Sep;12(3):599-607. doi: 10.1007/s40487-024-00294-3. Epub 2024 Aug 4. Oncol Ther. 2024. PMID: 39097531 Free PMC article.
-
Real-world outcomes of first-line maintenance niraparib monotherapy in patients with epithelial ovarian cancer.Future Oncol. 2025 Jan;21(2):213-219. doi: 10.1080/14796694.2024.2441654. Epub 2025 Jan 17. Future Oncol. 2025. PMID: 39819260 Free PMC article.
-
Real-world post-2020 first-line maintenance treatment patterns in patients with advanced ovarian cancer in the US.Future Oncol. 2025 Aug;21(19):2495-2503. doi: 10.1080/14796694.2025.2526273. Epub 2025 Jul 14. Future Oncol. 2025. PMID: 40654327 Free PMC article.
-
Ovarian Cancer Retrospective European (O'CaRE) study: first-line outcomes by number of risk factors for progression.Future Oncol. 2024 Dec;20(40):3409-3419. doi: 10.1080/14796694.2024.2402217. Epub 2024 Oct 24. Future Oncol. 2024. PMID: 39445504 Free PMC article.
References
-
- American Cancer Society. Key statistics for ovarian cancer. 2021. https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. Accessed 4 Dec 2022.
-
- Matsuo K, Bond VK, Eno ML, Im DD, Rosenshein NB. Low drug resistance to both platinum and taxane chemotherapy on an in vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian and peritoneal cancer. Int J Cancer. 2009;125:2721–2727. doi: 10.1002/ijc.24654. - DOI - PubMed
-
- Tapia G, Diaz-Padilla I. Molecular mechanisms of platinum resistance in ovarian cancer. In: Diaz-Padilla I, editor. Ovarian cancer: a clinical and translational update. InTech; 2013.
LinkOut - more resources
Full Text Sources
Research Materials

