Oral anticoagulants and concurrent rifampin administration in tuberculosis patients with non-valvular atrial fibrillation
- PMID: 37016321
- PMCID: PMC10074893
- DOI: 10.1186/s12872-023-03212-z
Oral anticoagulants and concurrent rifampin administration in tuberculosis patients with non-valvular atrial fibrillation
Abstract
Background: Evidence and guidelines for Non-vitamin K antagonist oral anticoagulants (NOACs) use when prescribing concurrent rifampin for tuberculosis treatment in patients with non-valvular atrial fibrillation (NVAF) are limited.
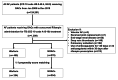
Methods: Using the Korean National Health Insurance Service database from January 2009 to December 2018, we performed a population-based retrospective cohort study to assess the net adverse clinical events (NACE), a composite of ischemic stroke or systemic embolism and major bleeding, of NOACs compared with warfarin among NVAF patients taking concurrent rifampin administration for tuberculosis treatment. After a propensity matching score (PSM) analysis, Cox proportional hazards regression was performed in matched cohorts to investigate the clinical outcomes.
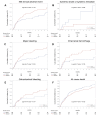
Results: Of the 735 consecutive patients selected, 465 (63.3%) received warfarin and 270 (36.7%) received NOACs. Among 254 pairs of patients after PSM, the crude incidence rate of NACE was 25.6 in NOAC group and 32.8 per 100 person-years in warfarin group. There was no significant difference between NOAC and warfarin use in NACE (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.48-1.14; P = 0.172). Major bleeding was the main driver of NACE, and NOAC use was associated with a statistically significantly lower risk of major bleeding than that with warfarin use (HR, 0.63; 95% CI, 0.40-1.00; P = 0.0499).
Conclusions: In our population-based study, there was no statically significant difference in the occurrence of NACE between NOAC and warfarin use. NOAC use may be associated with a lower risk of major bleeding than that with warfarin use.
Keywords: Anticoagulation; Atrial fibrillation; Drug-drug interactions; Rifampin; Tuberculosis.
© 2023. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Korea Centers for Disease Control and Prevention. Annual Report on the Notified Tuberculosis in Korea., 2019. Cheongju, Korea: Korea Centers for Disease Control and Prevention; 2020. Available from https://tbzero.kdca.go.kr/tbzero/board/boardView.do [Accessed 10 Sep 2021].
-
- Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official american thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:e1–e33. doi: 10.1093/cid/ciw694. - DOI - PubMed
-
- Fiore M, Maraolo AE, Chiodini P, Cerchione C, Gentile I, Borgia G, et al. Is anticoagulation with novel oral Anticoagulants an effective treatment for tuberculosis patients not achieving a therapeutic range with vitamin K antagonists? A systematic review. Cardiovasc Hematol Disord Drug Targets. 2017;17:105–10. doi: 10.2174/1871529X17666170703115545. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

