Cardiometabolic Monitoring and Sociodemographic and Clinical Characteristics of Youths Prescribed Antipsychotic Medications
- PMID: 37016828
- PMCID: PMC10539018
- DOI: 10.1176/appi.ps.20220151
Cardiometabolic Monitoring and Sociodemographic and Clinical Characteristics of Youths Prescribed Antipsychotic Medications
Abstract
Objective: This study examined time trends and patient characteristics related to guideline-recommended cardiometabolic risk factor monitoring among youths treated with antipsychotic medications.
Methods: This observational study assessed participant sociodemographic and clinical characteristics and year of antipsychotic medication initiation, with receipt of glycemic and lipid testing within 2 years of initiation as the primary outcome. Electronic health records and pharmacy data from Kaiser Permanente Northern California for 4,568 youths (ages 10-21 years) who began antipsychotic medication treatment during 2013-2017 were included.
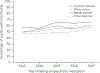
Results: Mean±SD age of the sample was 17.0±3.0 years, 52% were male, and 50% were Asian American, Native Hawaiian, or Pacific Islander; Black; Latino; or another or unknown race-ethnicity. Overall, 54% of the sample completed glycemic and lipid monitoring within 2 years of medication initiation (41% within 1 year). With each study year, monitoring rates increased by 5% in this cohort, after the analyses were adjusted for participant factors (p=0.001). In the fully adjusted analysis, youths with a psychotic disorder were 23% more likely to receive cardiometabolic monitoring than those without a psychotic disorder or bipolar disorder (p<0.001). Monitoring was also more common among younger versus older adolescents and among those with risperidone (vs. quetiapine) medication, obesity, or more frequent use of outpatient health care. Youths with (vs. without) substance use disorder were 19% less likely to complete monitoring (p<0.001).
Conclusions: Cardiometabolic monitoring increased modestly over time, but close to half of the studied youths did not receive glycemic or lipid testing. Additional clinical strategies may be needed to increase monitoring overall and among harder-to-reach youth subgroups.
Keywords: Antipsychotics; Cardiometabolic testing; Child psychiatry; Quality of care; Treatment guidelines.
Conflict of interest statement
The authors report no financial relationships with commercial interests.
Figures
References
-
- Olfson M, King M, Schoenbaum M: Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry 2015; 72:867–874 - PubMed
-
- Olfson M, Blanco C, Liu S-M, et al. : National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Arch Gen Psychiatry 2012; 69:1247–1256 - PubMed
-
- Pringsheim T, Lam D, Ching H, et al. : Metabolic and neurological complications of second-generation antipsychotic use in children: a systematic review and meta-analysis of randomized controlled trials. Drug Saf 2011; 34:651–668 - PubMed
-
- Fraguas D, Correll CU, Merchán-Naranjo J, et al. : Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: comprehensive review of prospective head-to-head and placebo-controlled comparisons. Eur Neuropsychopharmacol 2011; 21:621–645 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

