Immunogenicity and Safety of Hepatitis B Virus (HBV) Vaccine With a Toll-Like Receptor 9 Agonist Adjuvant in HBV Vaccine-Naïve People With Human Immunodeficiency Virus
- PMID: 37017075
- PMCID: PMC10681652
- DOI: 10.1093/cid/ciad201
Immunogenicity and Safety of Hepatitis B Virus (HBV) Vaccine With a Toll-Like Receptor 9 Agonist Adjuvant in HBV Vaccine-Naïve People With Human Immunodeficiency Virus
Abstract
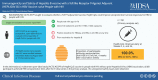
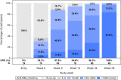
In this international, multicenter open-label study (ACTG A5379) of HepB-CpG vaccine in people with human immunodeficiency virus (HIV) without prior hepatitis B virus (HBV) vaccination, all 68 participants achieved HBV seroprotective titers after the 3-dose series in the primary analysis. No unexpected safety issues were observed.
Keywords: HIV; HepB-CpG; Heplisav-B; hepatitis B; vaccine.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. K. M. M. has received research funding paid to her institution from Gilead Sciences. A. A. has received research grants from Gilead Sciences, GlaxoSmithKline, MSD, travel support for meetings from Gilead Science, and has received speaker honoraria from Gilead Sciences, ViiV Healthcare, and GlaxoSmithKline. K. J. V. has been a consultant to ViiV Healthcare, Theratechnologies, and Gilead Sciences and has research funding from Theratechnologies Inc. J. C. P. has had research support paid to her institution: Gilead Sciences, Merck, AbbVie, Zydus, Genentech, and Vir. K. E. S. has had research support paid to his institution from AbbVie, Abbott Laboratories, Gilead, BMS, Inovio, Merck, Zydus, Intercept, Helio and Astra Zeneca. He also reports royalities or licenses from UpToDate, consulting fees from Gilead, Theratechnologies, Axcella, MedPace, and Inovio (paid to author), stock or stock options from Exxon-Mobil, McDonalds, and Pepsico, and has served on DSMBs managed by Pliant, Horizon, ACI, Inovio, and MedPace. H. P. reports a grant from CNPq-Brazil (grant number 445957/2020-4) for an HCV treatment clinical trial. T. U. reports grants or contracts from NIH/NHLBI (REPRIEVE DCC grant number U01 HL23339), subcontract from KOWA Pharmaceutical via MGH in support of REPRIEVE-EU, and NIH/NIA subcontract from UC Denver (R01 AG054366). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Halperin SA, Brian W, Curtis C, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18–55 years of age. Vaccine 2012; 30:2556–63. - PubMed
-
- Heyward WL, Kyle M, Blumenau J, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40–70 years of age. Vaccine 2013; 31:5300–5. - PubMed
-
- Jackson S, Lentino J, Kopp J, et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a Toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine 2018; 36:668–74. - PubMed
-
- Janssen RS, Mangoo-Karim R, Pergola PE, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in patients with chronic kidney disease. Vaccine 2013; 31:5306–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069501/AI/NIAID NIH HHS/United States
- UM1 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI069456/AI/NIAID NIH HHS/United States
- U01 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States

