The OPAQUE1/DISCORDIA2 myosin XI is required for phragmoplast guidance during asymmetric cell division in maize
- PMID: 37017144
- PMCID: PMC10291028
- DOI: 10.1093/plcell/koad099
The OPAQUE1/DISCORDIA2 myosin XI is required for phragmoplast guidance during asymmetric cell division in maize
Abstract
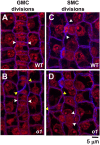
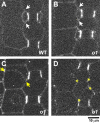
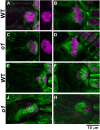
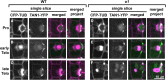
Formative asymmetric divisions produce cells with different fates and are critical for development. We show the maize (Zea mays) myosin XI protein, OPAQUE1 (O1), is necessary for asymmetric divisions during maize stomatal development. We analyzed stomatal precursor cells before and during asymmetric division to determine why o1 mutants have abnormal division planes. Cell polarization and nuclear positioning occur normally in the o1 mutant, and the future site of division is correctly specified. The defect in o1 becomes apparent during late cytokinesis, when the phragmoplast forms the nascent cell plate. Initial phragmoplast guidance in o1 is normal; however, as phragmoplast expansion continues o1 phragmoplasts become misguided. To understand how O1 contributes to phragmoplast guidance, we identified O1-interacting proteins. Maize kinesins related to the Arabidopsis thaliana division site markers PHRAGMOPLAST ORIENTING KINESINs (POKs), which are also required for correct phragmoplast guidance, physically interact with O1. We propose that different myosins are important at multiple steps of phragmoplast expansion, and the O1 actin motor and POK-like microtubule motors work together to ensure correct late-stage phragmoplast guidance.
© American Society of Plant Biologists 2023. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures





Comment in
-
Charting the course of asymmetric cell division in maize: The crucial role of OPAQUE1 in guiding the phragmoplast.Plant Cell. 2023 Jun 26;35(7):2447-2448. doi: 10.1093/plcell/koad104. Plant Cell. 2023. PMID: 37032442 Free PMC article. No abstract available.
References
-
- Allsman LA, Dieffenbacher RN, Rasmussen CG. Glue impressions of maize leaves and their use in classifying mutants. Bio-101. 2019:e3209. 10.21769/BioProtoc.3209 - DOI
-
- Apostolakos P, Livanos P, Giannoutsou E, Panteris E, Galatis B. The intracellular and intercellular cross-talk during subsidiary cell formation in Zea mays: existing and novel components orchestrating cell polarization and asymmetric division. Ann Bot. 2018:122(5):679–696. 10.1093/aob/mcx193 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

