Intragranuloma Accumulation and Inflammatory Differentiation of Neutrophils Underlie Mycobacterial ESX-1-Dependent Immunopathology
- PMID: 37017530
- PMCID: PMC10127687
- DOI: 10.1128/mbio.02764-22
Intragranuloma Accumulation and Inflammatory Differentiation of Neutrophils Underlie Mycobacterial ESX-1-Dependent Immunopathology
Abstract
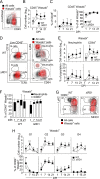
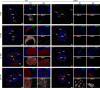
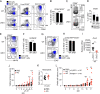
The conserved ESX-1 type VII secretion system is a major virulence determinant of pathogenic mycobacteria, including Mycobacterium tuberculosis and Mycobacterium marinum. ESX-1 is known to interact with infected macrophages, but its potential roles in regulating other host cells and immunopathology have remained largely unexplored. Using a murine M. marinum infection model, we identify neutrophils and Ly6C+MHCII+ monocytes as the main cellular reservoirs for the bacteria. We show that ESX-1 promotes intragranuloma accumulation of neutrophils and that neutrophils have a previously unrecognized required role in executing ESX-1-mediated pathology. To explore if ESX-1 also regulates the function of recruited neutrophils, we performed a single-cell RNA-sequencing analysis that indicated that ESX-1 drives newly recruited uninfected neutrophils into an inflammatory phenotype via an extrinsic mechanism. In contrast, monocytes restricted the accumulation of neutrophils and immunopathology, demonstrating a major host-protective function for monocytes specifically by suppressing ESX-1-dependent neutrophilic inflammation. Inducible nitric oxide synthase (iNOS) activity was required for the suppressive mechanism, and we identified Ly6C+MHCII+ monocytes as the main iNOS-expressing cell type in the infected tissue. These results suggest that ESX-1 mediates immunopathology by promoting neutrophil accumulation and phenotypic differentiation in the infected tissue, and they demonstrate an antagonistic interplay between monocytes and neutrophils by which monocytes suppress host-detrimental neutrophilic inflammation. IMPORTANCE The ESX-1 type VII secretion system is required for virulence of pathogenic mycobacteria, including Mycobacterium tuberculosis. ESX-1 interacts with infected macrophages, but its potential roles in regulating other host cells and immunopathology have remained largely unexplored. We demonstrate that ESX-1 promotes immunopathology by driving intragranuloma accumulation of neutrophils, which upon arrival adopt an inflammatory phenotype in an ESX-1-dependent manner. In contrast, monocytes limited the accumulation of neutrophils and neutrophil-mediated pathology via an iNOS-dependent mechanism, suggesting a major host-protective function for monocytes specifically by restricting ESX-1-dependent neutrophilic inflammation. These findings provide insight into how ESX-1 promotes disease, and they reveal an antagonistic functional relationship between monocytes and neutrophils that might regulate immunopathology not only in mycobacterial infection but also in other infections as well as in inflammatory conditions and cancer.
Keywords: ESX-1 type VII secretion; granuloma; host-pathogen interactions; iNOS; immunopathology; monocytes; mycobacterial pathogenesis; neutrophils; single-cell RNA-seq.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Stinear TP, Seemann T, Harrison PF, Jenkin GA, Davies JK, Johnson PD, Abdellah Z, Arrowsmith C, Chillingworth T, Churcher C, Clarke K, Cronin A, Davis P, Goodhead I, Holroyd N, Jagels K, Lord A, Moule S, Mungall K, Norbertczak H, Quail MA, Rabbinowitsch E, Walker D, White B, Whitehead S, Small PL, Brosch R, Ramakrishnan L, Fischbach MA, Parkhill J, Cole ST. 2008. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res 18:729–741. doi: 10.1101/gr.075069.107. - DOI - PMC - PubMed
-
- Wassermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, Schmid-Burgk JL, Schmidt T, Hornung V, Cole ST, Ablasser A. 2015. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe 17:799–810. doi: 10.1016/j.chom.2015.05.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
