Salmonella Invasion Is Controlled by Competition among Intestinal Chemical Signals
- PMID: 37017539
- PMCID: PMC10127606
- DOI: 10.1128/mbio.00012-23
Salmonella Invasion Is Controlled by Competition among Intestinal Chemical Signals
Abstract
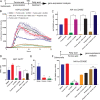
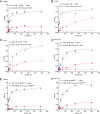
The intestine is a complex, ever-changing environment replete with an array of signaling molecules. To colonize such a complex organ, pathogens have adapted to utilize specific cues from the local environment to intricately regulate the expression of their virulence determinants. Salmonella preferentially colonizes the distal ileum, a niche enriched in the metabolite formic acid. Here, we show that the relatively higher concentration of this metabolite in the distal ileum prevents other signals from repressing Salmonella invasion in that region. We show that imported and unmetabolized formic acid functions as a cytoplasmic signal that competitively binds to HilD, the master transcriptional regulator of Salmonella invasion, thus preventing repressive fatty acids from binding to the protein. This results in an increased lifetime of HilD and subsequent derepression of invasion genes. This study demonstrates an important mechanism by which Salmonella utilizes competition among signals in the gut to its advantage as a pathogen. IMPORTANCE Enteric pathogens acutely sense their environment for signals to regulate their virulence functions. We demonstrate here that the enteric pathogen Salmonella utilizes the competition among certain regional intestinal constituents to modulate its virulence determinants in that region. We show that the high concentration of formic acid in the ileum outcompetes other signals and triggers the activation of virulence genes in the ileum. This study shows a delicate spatial and temporal mechanism by which enteric pathogens may utilize the competition among environmental cues to optimize their pathogenicity.
Keywords: Salmonella; enteric pathogens; fatty acids; host-pathogen interactions; infectious disease; pathogenesis mechanisms; virulence regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

