Cost-effectiveness of Hepatitis C virus self-testing in four settings
- PMID: 37018166
- PMCID: PMC10075433
- DOI: 10.1371/journal.pgph.0001667
Cost-effectiveness of Hepatitis C virus self-testing in four settings
Abstract
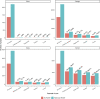
Globally, there are approximately 58 million people with chronic hepatitis C virus infection (HCV) but only 20% have been diagnosed. HCV self-testing (HCVST) could reach those who have never been tested and increase uptake of HCV testing services. We compared cost per HCV viraemic diagnosis or cure for HCVST versus facility-based HCV testing services. We used a decision analysis model with a one-year time horizon to examine the key drivers of economic cost per diagnosis or cure following the introduction of HCVST in China (men who have sex with men), Georgia (men 40-49 years), Viet Nam (people who inject drugs, PWID), and Kenya (PWID). HCV antibody (HCVAb) prevalence ranged from 1%-60% across settings. Model parameters in each setting were informed by HCV testing and treatment programmes, HIV self-testing programmes, and expert opinion. In the base case, we assume a reactive HCVST is followed by a facility-based rapid diagnostic test (RDT) and then nucleic acid testing (NAT). We assumed oral-fluid HCVST costs of $5.63/unit ($0.87-$21.43 for facility-based RDT), 62% increase in testing following HCVST introduction, 65% linkage following HCVST, and 10% replacement of facility-based testing with HCVST based on HIV studies. Parameters were varied in sensitivity analysis. Cost per HCV viraemic diagnosis without HCVST ranged from $35 2019 US dollars (Viet Nam) to $361 (Kenya). With HCVST, diagnosis increased resulting in incremental cost per diagnosis of $104 in Viet Nam, $163 in Georgia, $587 in Kenya, and $2,647 in China. Differences were driven by HCVAb prevalence. Switching to blood-based HCVST ($2.25/test), increasing uptake of HCVST and linkage to facility-based care and NAT testing, or proceeding directly to NAT testing following HCVST, reduced the cost per diagnosis. The baseline incremental cost per cure was lowest in Georgia ($1,418), similar in Viet Nam ($2,033), and Kenya ($2,566), and highest in China ($4,956). HCVST increased the number of people tested, diagnosed, and cured, but at higher cost. Introducing HCVST is more cost-effective in populations with high prevalence.
Copyright: © 2023 Walker et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
EI and SS are current employees of FIND, EF is a former employee of FIND. JGW and PV have received unrestricted research funding from Gilead Sciences unrelated to this research. All authors declare no other conflicts of interest. The views expressed in this manuscript are those of the authors and do not necessarily represent the official position, decisions, policy or views of the WHO or UNAIDS. There are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. Geneva: World Health Organization; 2016. 2016. Contract No.: WHO/HIV/2016.06.
-
- World Health Organization. Second round of the national pulse survey on continuity of essential health services during the COVID-19 pandemic. Interim report. 2021. Contract No.: WHO reference number: WHO/2019-nCoV/EHS_continuity/survey/2021.1.
-
- World Health Organization. Guidelines on hepatitis B and C testing. Geneva: World Health Organization; 2017.
-
- Easterbrook PJ WHO Guidelines Development Group. Who to test and how to test for chronic hepatitis C infection—2016 WHO testing guidance for low- and middle-income countries. J Hepatol. 2016;65(1 Suppl):S46–S66. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
