Catalytically Active Snake Venom PLA2 Enzymes: An Overview of Its Elusive Mechanisms of Reaction
- PMID: 37018514
- PMCID: PMC10150362
- DOI: 10.1021/acs.jmedchem.3c00097
Catalytically Active Snake Venom PLA2 Enzymes: An Overview of Its Elusive Mechanisms of Reaction
Abstract
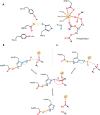
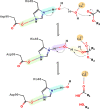
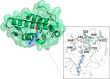
Snake venom-secreted phospholipase A2 (svPLA2) enzymes, both catalytically active and inactive, are a central component in envenoming. These are responsible for disrupting the cell membrane's integrity, inducing a wide range of pharmacological effects, such as the necrosis of the bitten limb, cardiorespiratory arrest, edema, and anticoagulation. Although extensively characterized, the reaction mechanisms of enzymatic svPLA2 are still to be thoroughly understood. This review presents and analyses the most plausible reaction mechanisms for svPLA2, such as the "single-water mechanism" or the "assisted-water mechanism" initially proposed for the homologous human PLA2. All of the mechanistic possibilities are characterized by a highly conserved Asp/His/water triad and a Ca2+ cofactor. The extraordinary increase in activity induced by binding to a lipid-water interface, known as "interfacial activation," critical for the PLA2s activity, is also discussed. Finally, a potential catalytic mechanism for the postulated noncatalytic PLA2-like proteins is anticipated.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




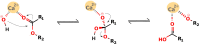

References
-
- Williams D. J.; Faiz M. A.; Abela-Ridder B.; Ainsworth S.; Bulfone T. C.; Nickerson A. D.; Habib A. G.; Junghanss T.; Fan H. W.; Turner M.; Harrison R. A.; Warrell D. A. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019, 13, e0007059 10.1371/journal.pntd.0007059. - DOI - PMC - PubMed
-
- Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; World Health Organization: Geneva, 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

