Patients' experiences with pre-test genetic counseling provided by breast cancer healthcare professionals: Results from a large prospective multicenter study
- PMID: 37018966
- PMCID: PMC10122003
- DOI: 10.1016/j.breast.2023.03.017
Patients' experiences with pre-test genetic counseling provided by breast cancer healthcare professionals: Results from a large prospective multicenter study
Abstract
Background: Pre-test genetic counseling of patients with breast cancer is increasingly being offered by non-genetic healthcare professionals. We aimed to evaluate the experiences of patients with breast cancer receiving pre-test genetic counseling from a non-genetic healthcare professional (i.e., surgeon or nurse).
Methods: Patients who were diagnosed with breast cancer and received pre-test counseling from their surgeon or nurse (mainstream group), and patients who received pre-test counseling from a clinical geneticist (usual care group) were invited to participate in our multicenter study. Between September 2019 and December 2021, patients received a questionnaire after pre-test counseling (T0) and four weeks after receiving their test results (T1) to evaluate psychosocial outcomes, knowledge, discussed topics and satisfaction.
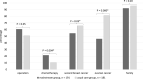
Results: We included 191 patients in our mainstream and 183 patients in our usual care group and received, respectively 159 and 145 follow-up questionnaires. Levels of distress and decisional regret were comparable in both groups. Decisional conflict was higher in our mainstream group (p = 0.01), but only 7% had clinically relevant decisional conflict (vs 2% in usual care group). The possible implications of a genetic test on (secondary) breast or ovarian cancer risks were less frequently discussed in our mainstream group (p = 0.03 and p = 0.000, respectively). In both groups knowledge about genetics was comparable, satisfaction was high and the majority of patients in both groups preferred to give both verbal and written consent for genetic testing.
Conclusion: Mainstreamed genetic care provides sufficient information for the majority of breast cancer patients to decide about genetic testing with minimal distress.
Keywords: Breast cancer; Genetic counseling; Mainstream genetic testing; Patients' perspectives; Psychosocial outcomes; Satisfaction.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declarations of competing interest The authors have no conflicts of interest to declare.
Figures

References
-
- Paluch-Shimon S., Cardoso F., Sessa C., Balmana J., Cardoso M.J., Gilbert F., et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103–v110. - PubMed
-
- Tung N.M., Boughey J.C., Pierce L.J., Robson M.E., Bedrosian I., Dietz J.R., et al. Management of hereditary breast cancer: American society of clinical oncology, American society for radiation oncology, and society of surgical oncology guideline. J Clin Oncol. 2020;38(18):2080–2106. - PubMed
-
- Gradishar W.J., Anderson B.O., Abraham J., Aft R., Agnese D., Allison K.H., et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020 Apr;18(4):452–478. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

