The Lymphatic System in Kidney Disease
- PMID: 37019177
- PMCID: PMC10371377
- DOI: 10.34067/KID.0000000000000120
The Lymphatic System in Kidney Disease
Abstract
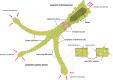
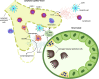
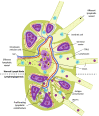
The high-capacity vessels of the lymphatic system drain extravasated fluid and macromolecules from nearly every part of the body. However, far from merely a passive conduit for fluid removal, the lymphatic system also plays a critical and active role in immune surveillance and immune response modulation through the presentation of fluid, macromolecules, and trafficking immune cells to surveillance cells in regional draining lymph nodes before their return to the systemic circulation. The potential effect of this system in numerous disease states both within and outside of the kidney is increasingly being explored for their therapeutic potential. In the kidneys, the lymphatics play a critical role in both fluid and macromolecule removal to maintain oncotic and hydrostatic pressure gradients for normal kidney function, as well as in shaping kidney immunity, and potentially in balancing physiological pathways that promote healthy organ maintenance and responses to injury. In many states of kidney disease, including AKI, the demand on the preexisting lymphatic network increases for clearance of injury-related tissue edema and inflammatory infiltrates. Lymphangiogenesis, stimulated by macrophages, injured resident cells, and other drivers in kidney tissue, is highly prevalent in settings of AKI, CKD, and transplantation. Accumulating evidence points toward lymphangiogenesis being possibly harmful in AKI and kidney allograft rejection, which would potentially position lymphatics as another target for novel therapies to improve outcomes. However, the extent to which lymphangiogenesis is protective rather than maladaptive in the kidney in various settings remains poorly understood and thus an area of active research.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

