An early, reversible cholesterolgenic etiology of diet-induced insulin resistance
- PMID: 37019209
- PMCID: PMC10114231
- DOI: 10.1016/j.molmet.2023.101715
An early, reversible cholesterolgenic etiology of diet-induced insulin resistance
Abstract
Objective: A buildup of skeletal muscle plasma membrane (PM) cholesterol content in mice occurs within 1 week of a Western-style high-fat diet and causes insulin resistance. The mechanism driving this cholesterol accumulation and insulin resistance is not known. Promising cell data implicate that the hexosamine biosynthesis pathway (HBP) triggers a cholesterolgenic response via increasing the transcriptional activity of Sp1. In this study we aimed to determine whether increased HBP/Sp1 activity represented a preventable cause of insulin resistance.
Methods: C57BL/6NJ mice were fed either a low-fat (LF, 10% kcal) or high-fat (HF, 45% kcal) diet for 1 week. During this 1-week diet the mice were treated daily with either saline or mithramycin-A (MTM), a specific Sp1/DNA-binding inhibitor. A series of metabolic and tissue analyses were then performed on these mice, as well as on mice with targeted skeletal muscle overexpression of the rate-limiting HBP enzyme glutamine-fructose-6-phosphate-amidotransferase (GFAT) that were maintained on a regular chow diet.
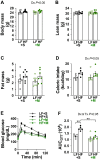
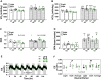
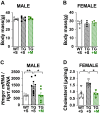
Results: Saline-treated mice fed this HF diet for 1 week did not have an increase in adiposity, lean mass, or body mass while displaying early insulin resistance. Consistent with an HBP/Sp1 cholesterolgenic response, Sp1 displayed increased O-GlcNAcylation and binding to the HMGCR promoter that increased HMGCR expression in skeletal muscle from saline-treated HF-fed mice. Skeletal muscle from these saline-treated HF-fed mice also showed a resultant elevation of PM cholesterol with an accompanying loss of cortical filamentous actin (F-actin) that is essential for insulin-stimulated glucose transport. Treating these mice daily with MTM during the 1-week HF diet fully prevented the diet-induced Sp1 cholesterolgenic response, loss of cortical F-actin, and development of insulin resistance. Similarly, increases in HMGCR expression and cholesterol were measured in muscle from GFAT transgenic mice compared to age- and weight-match wildtype littermate control mice. In the GFAT Tg mice we found that these increases were alleviated by MTM.
Conclusions: These data identify increased HBP/Sp1 activity as an early mechanism of diet-induced insulin resistance. Therapies targeting this mechanism may decelerate T2D development.
Keywords: Cholesterol; Insulin resistance; Membrane; Skeletal muscle.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures

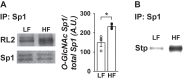


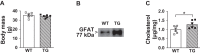
References
-
- Norton L., Shannon C., Gastaldelli A., DeFronzo R.A. Insulin: the master regulator of glucose metabolism. Metabolism. 2022;129 - PubMed
-
- Grice B.A., Barton K.J., Covert J.D., Kreilach A.M., Tackett L., Brozinick J.T., et al. Excess membrane cholesterol is an early contributing reversible aspect of skeletal muscle insulin resistance in C57BL/6NJ mice fed a Western-style high-fat diet. Am J Physiol Endocrinol Metab. 2019;317(2):E362–E373. - PMC - PubMed
-
- Tsakiridis T., Vranic M., Klip A. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J Biol Chem. 1994;269(47):29934–29942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

