Passive Recording of Bioelectrical Signals from Non-Excitable Cells by Fluorescent Mirroring
- PMID: 37019439
- PMCID: PMC10141418
- DOI: 10.1021/acs.nanolett.2c05053
Passive Recording of Bioelectrical Signals from Non-Excitable Cells by Fluorescent Mirroring
Abstract
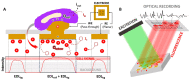
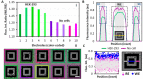
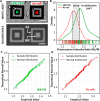
Bioelectrical variations trigger different cell responses, including migration, mitosis, and mutation. At the tissue level, these actions result in phenomena such as wound healing, proliferation, and pathogenesis. Monitoring these mechanisms dynamically is highly desirable in diagnostics and drug testing. However, existing technologies are invasive: either they require physical access to the intracellular compartments, or they imply direct contact with the cellular medium. Here, we present a novel approach for the passive recording of electrical signals from non-excitable cells adhering to 3D microelectrodes, based on optical mirroring. Preliminary results yielded a fluorescence intensity output increase of the 5,8% in the presence of a HEK-293 cell on the electrode compared to bare microelectrodes. At present, this technology may be employed to evaluate cell-substrate adhesion and monitor cell proliferation. Further refinements could allow extrapolating quantitative data on surface charges and resting potential to investigate the electrical phenomena involved in cell migration and cancer progression.
Keywords: HEK-293; bioelectricity; cell−surface adhesion; fluorescence; non−excitable cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Cui B. X.; Jahed Z.; Yang Y.; Tsai C. T. Intracellular recording of cardiac action potentials via membrane electroporation. Biophys. J. 2022, 121 (3), 304a.10.1016/j.bpj.2021.11.1241. - DOI
-
- Dipalo M.; Rastogi S. K.; Matino L.; Garg R.; Bliley J.; Iachetta G.; Melle G.; Shrestha R.; Shen S.; Santoro F.; et al. Intracellular action potential recordings from cardiomyocytes by ultrafast pulsed laser irradiation of fuzzy graphene microelectrodes. Sci. Adv. 2021, 7 (15), eadb5175.10.1126/sciadv.abd5175. - DOI - PMC - PubMed
-
- Cervera J.; Pai V. P.; Levin M.; Mafe S. From non-excitable single-cell to multicellular bioelectrical states supported by ion channels and gap junction proteins: Electrical potentials as distributed controllers. Prog. Biophys. Mol. Biol. 2019, 149, 39–53. 10.1016/j.pbiomolbio.2019.06.004. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

