Comparing biparametric to multiparametric MRI in the diagnosis of clinically significant prostate cancer in biopsy-naive men (PRIME): a prospective, international, multicentre, non-inferiority within-patient, diagnostic yield trial protocol
- PMID: 37019486
- PMCID: PMC10083803
- DOI: 10.1136/bmjopen-2022-070280
Comparing biparametric to multiparametric MRI in the diagnosis of clinically significant prostate cancer in biopsy-naive men (PRIME): a prospective, international, multicentre, non-inferiority within-patient, diagnostic yield trial protocol
Abstract
Introduction: Prostate MRI is a well-established tool for the diagnostic work-up for men with suspected prostate cancer (PCa). Current recommendations advocate the use of multiparametric MRI (mpMRI), which is composed of three sequences: T2-weighted sequence (T2W), diffusion-weighted sequence (DWI) and dynamic contrast-enhanced sequence (DCE). Prior studies suggest that a biparametric MRI (bpMRI) approach, omitting the DCE sequences, may not compromise clinically significant cancer detection, though there are limitations to these studies, and it is not known how this may affect treatment eligibility. A bpMRI approach will reduce scanning time, may be more cost-effective and, at a population level, will allow more men to gain access to an MRI than an mpMRI approach.
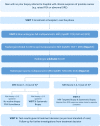
Methods: Prostate Imaging Using MRI±Contrast Enhancement (PRIME) is a prospective, international, multicentre, within-patient diagnostic yield trial assessing whether bpMRI is non-inferior to mpMRI in the diagnosis of clinically significant PCa. Patients will undergo the full mpMRI scan. Radiologists will be blinded to the DCE and will initially report the MRI using only the bpMRI (T2W and DWI) sequences. They will then be unblinded to the DCE sequence and will then re-report the MRI using the mpMRI sequences (T2W, DWI and DCE). Men with suspicious lesions on either bpMRI or mpMRI will undergo prostate biopsy. The main inclusion criteria are men with suspected PCa, with a serum PSA of ≤20 ng/mL and without prior prostate biopsy. The primary outcome is the proportion of men with clinically significant PCa detected (Gleason score ≥3+4 or Gleason grade group ≥2). A sample size of at least 500 patients is required. Key secondary outcomes include the proportion of clinically insignificant PCa detected and treatment decision.
Ethics and dissemination: Ethical approval was obtained from the National Research Ethics Committee West Midlands, Nottingham (21/WM/0091). Results of this trial will be disseminated through peer-reviewed publications. Participants and relevant patient support groups will be informed about the results of the trial.
Trial registration number: NCT04571840.
Keywords: Magnetic resonance imaging; Prostate disease; Protocols & guidelines; RADIOLOGY & IMAGING; Urological tumours.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: AN is an academic clinical fellow funded by the National Institute for Health and Care Research. PK is an academic clinical fellow funded by the National Institute for Health and Care Research and The Urology Foundation. FG is a recipient of the 2020 Young Investigator Award (20YOUN15) funded by the Prostate Cancer Foundation / CRIS Cancer Foundation. SP is supported by the National Institute of Health and Care Research (NIHR), UCLH and UCL Biomedical Research Centre. ME receives research support from the National Institute of Health and Care Research (NIHR), UCLH and UCL Biomedical Research Centre. YT is funded by a UK NIHR Postdoctoral Fellowship and supported by the NIHR Birmingham Biomedical Research Centre. CMM is an NIHR Research Professor, and receives grants from MRC, CRUK, Movember, and Prostate Cancer UK. VK is funded by Prostate Cancer UK and The John Black Charitable Foundation. He receives speaker fees from the European Association of Urology, Singapore Urology Association, The Clinical Comms Group and Got IT consulting SL. All authors declare that there are no conflicts of interest. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, NIHR, or the Department of Health and Social Care.
Figures
References
-
- Mottet N, Cornford P, Van Den Bergh R, et al. . EAU guidelines: prostate cancer. European Association of Urology, Arnhem, The Netherlands; 2021. Available: https://uroweb.org/guideline/prostate-cancer/
-
- National prostate cancer audit. Annual Report 2019; Available: https://www.npca.org.uk/reports/npca-annual-report-2019/
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
