Opioids for procedural pain in neonates
- PMID: 37019853
- PMCID: PMC10075060
- DOI: 10.1002/14651858.CD015056.pub2
Opioids for procedural pain in neonates
Update in
-
Opioids for procedural pain in neonates.Cochrane Database Syst Rev. 2023 Jun 23;6(6):CD015056. doi: 10.1002/14651858.CD015056.pub3. Cochrane Database Syst Rev. 2023. PMID: 37350685 Free PMC article. Review.
Abstract
Background: Neonates might be exposed to numerous painful procedures due to diagnostic reasons, therapeutic interventions, or surgical procedures. Options for pain management include opioids, non-pharmacological interventions, and other drugs. Morphine, fentanyl, and remifentanil are the opioids most often used in neonates. However, negative impact of opioids on the structure and function of the developing brain has been reported.
Objectives: To evaluate the benefits and harms of opioids in term or preterm neonates exposed to procedural pain, compared to placebo or no drug, non-pharmacological intervention, other analgesics or sedatives, other opioids, or the same opioid administered by a different route.
Search methods: We used standard, extensive Cochrane search methods. The latest search date was December 2021.
Selection criteria: We included randomized controlled trials conducted in preterm and term infants of a postmenstrual age (PMA) up to 46 weeks and 0 days exposed to procedural pain where opioids were compared to 1) placebo or no drug; 2) non-pharmacological intervention; 3) other analgesics or sedatives; 4) other opioids; or 5) the same opioid administered by a different route.
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were pain assessed with validated methods and any harms. We used a fixed-effect model with risk ratio (RR) for dichotomous data and mean difference (MD) for continuous data, and their confidence intervals (CI). We used GRADE to assess the certainty of the evidence for each outcome.
Main results: We included 13 independent studies (enrolling 823 newborn infants): seven studies compared opioids to no treatment or placebo (the main comparison in this review), two studies to oral sweet solution or non-pharmacological intervention, and five studies (of which two were part of the same study) to other analgesics and sedatives. All studies were performed in a hospital setting. Opioids compared to placebo or no drug Compared to placebo, opioids probably reduce pain score assessed with the Premature Infant Pain Profile (PIPP)/PIPP-Revised (PIPP-R) scale during the procedure (MD -2.58, 95% CI -3.12 to -2.03; 199 participants, 3 studies; moderate-certainty evidence); may reduce Neonatal Infant Pain Scale (NIPS) during the procedure (MD -1.97, 95% CI -2.46 to -1.48; 102 participants, 2 studies; low-certainty evidence); and may result in little to no difference in pain score assessed with the Douleur Aiguë du Nouveau-né (DAN) scale one to two hours after the procedure (MD -0.20, 95% CI -2.21 to 1.81; 42 participants, 1 study; low-certainty evidence). The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP-R scale up to 30 minutes after the procedure (MD 0.14, 95% CI -0.17 to 0.45; 123 participants, 2 studies; very low-certainty evidence) or one to two hours after the procedure (MD -0.83, 95% CI -2.42 to 0.75; 54 participants, 2 studies; very low-certainty evidence). No studies reported any harms. The evidence is very uncertain about the effect of opioids on episodes of bradycardia (RR 3.19, 95% CI 0.14 to 72.69; 172 participants, 3 studies; very low-certainty evidence). Opioids may result in an increase in episodes of apnea compared to placebo (RR 3.15, 95% CI 1.08 to 9.16; 199 participants, 3 studies; low-certainty evidence). The evidence is very uncertain about the effect of opioids on episodes of hypotension (RR not estimable, risk difference 0.00, 95% CI -0.06 to 0.06; 88 participants, 2 studies; very low-certainty evidence). No studies reported parent satisfaction with care provided in the neonatal intensive care unit (NICU). Opioids compared to non-pharmacological intervention The evidence is very uncertain about the effect of opioids on pain score assessed with the Crying Requires oxygen Increased vital signs Expression Sleep (CRIES) scale during the procedure when compared to facilitated tucking (MD -4.62, 95% CI -6.38 to -2.86; 100 participants, 1 study; very low-certainty evidence) or sensorial stimulation (MD 0.32, 95% CI -1.13 to 1.77; 100 participants, 1 study; very low-certainty evidence). The other main outcomes were not reported. Opioids compared to other analgesics or sedatives The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP-R during the procedure (MD -0.29, 95% CI -1.58 to 1.01; 124 participants, 2 studies; very low-certainty evidence); up to 30 minutes after the procedure (MD -1.10, 95% CI -2.82 to 0.62; 12 participants, 1 study; very low-certainty evidence); and one to two hours after the procedure (MD -0.17, 95% CI -2.22 to 1.88; 12 participants, 1 study; very low-certainty evidence). No studies reported any harms. The evidence is very uncertain about the effect of opioids on episodes of apnea during (RR 3.27, 95% CI 0.85 to 12.58; 124 participants, 2 studies; very low-certainty evidence) and after the procedure (RR 2.71, 95% CI 0.11 to 64.96; 124 participants, 2 studies; very low-certainty evidence) and on hypotension (RR 1.34, 95% CI 0.32 to 5.59; 204 participants, 3 studies; very low-certainty evidence). The other main outcomes were not reported. We identified no studies comparing different opioids (e.g. morphine versus fentanyl) or different routes for administration of the same opioid (e.g. morphine enterally versus morphine intravenously).
Authors' conclusions: Compared to placebo, opioids probably reduce pain score assessed with PIPP/PIPP-R scale during the procedure; may reduce NIPS during the procedure; and may result in little to no difference in DAN one to two hours after the procedure. The evidence is very uncertain about the effect of opioids on pain assessed with other pain scores or at different time points. No studies reported if any harms occurred. The evidence is very uncertain about the effect of opioids on episodes of bradycardia or hypotension. Opioids may result in an increase in episodes of apnea. No studies reported parent satisfaction with care provided in the NICU. The evidence is very uncertain about the effect of opioids on any outcome when compared to non-pharmacological interventions or to other analgesics. We identified no studies comparing opioids to other opioids or comparing different routes of administration of the same opioid.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MK works as a physician in the Department of Pediatrics, Yokohama Municipal Citizens' Hospital.
EO works clinically (part‐time) as a registered nurse at Örebro University Hospital, and therefore cares for newborns exposed to procedural pain.
FB has started a neonatology residency in II Department of Neonatology in GPSK clinical hospital, Poznan University of Medical Sciences, Poznan, Poland.
MB is an Associate Editor for the Cochrane Neonatal Group. However, his participation in the editorial group has not impacted this review.
Figures
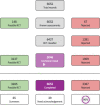
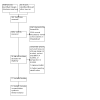
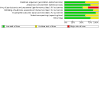

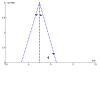
















References
References to studies included in this review
Carbajal 2005 {published data only}
-
- Carbajal R, Lenclen R, Jugie M, Paupe A, Barton B, Anand KS. Morphine does not alleviate acute pain in preterm neonates. Pediatric Research 2004;55:38. - PubMed
Cordero 1991 {published data only}
-
- Cordero L, Gardner D, O'Shaughnessy R. Low dose analgesia for broviac catheter placement (BCP). Pediatric Research 1990;27:203A.
Fallah 2016 {published data only}
-
- IRCT2014022616761N1. Effect of fentanyl on pain reduction. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT2014022616761N1 (first received 16 April 2014). [CENTRAL: CN-01863513]
Gitto 2012 {published data only}
-
- Aversa S, Marseglia L, Manfrida M, Reiter RJ, Gitto E. Stress response and procedural pain in the preterm newborn: the role of pharmacological and non-pharmacological treatments. Journal of Maternal-Fetal & Neonatal Medicine 2012;25:134. - PubMed
-
- Gitto E, Pellegrino S, Manfrida M, Aversa S, Trimarchi G, Barberi I, et al. Stress response and procedural pain in the preterm newborn: the role of pharmacological and non-pharmacological treatments. European Journal of Pediatrics 2012;171(6):927-33. [DOI: 10.1007/s00431-011-1655-7] [PMID: ] - DOI - PubMed
Hartley 2018 {published data only}
-
- EUCTR2014-003237-25-GB. Does morphine provide effective pain relief during painful procedures performed in newborn babies as part of their essential medical care? trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2014-003237-25-GB (first received 20 July 2015). [CENTRAL: CN-01844452]
-
- Hartley C, Moultrie F, Hoskin A, Green G, Monk V, Bell JL, et al. Analgesic efficacy and safety of morphine in the Procedural Pain in Premature Infants (Poppi) study: randomised placebo-controlled trial. Lancet 2018;392(10164):2595-605. [DOI: 10.1016/S0140-6736(18)31813-0] [PMID: ] - DOI - PMC - PubMed
-
- ISRCTN82342359. Is morphine an effective analgesic for procedural pain in infants? trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN82342359 (first received 29 July 2015). [CENTRAL: CN-01863765]
-
- Slater R, Hartley C, Moultrie F, Adams E, Juszczak E, Rogers R, et al, Poppi Trial Team. A blinded randomised placebo-controlled trial investigating the efficacy of morphine analgesia for procedural pain in infants: trial protocol. Wellcome Open Research 2016;1:7. [DOI: 10.12688/wellcomeopenres.10005.2] [PMID: ] - DOI - PMC - PubMed
Lago 2008 {published data only}
Madathil 2021a {published data only}
-
- Madathil S, Thomas D, Chandra P, Agarwal R, Sankar MJ, Thukral A, et al. 'NOPAIN-ROP' trial: intravenous fentanyl and intravenous ketamine for pain relief during laser photocoagulation for retinopathy of prematurity (ROP) in preterm infants: a randomised trial. BMJ Open 2021;11(9):e046235. [DOI: 10.1136/bmjopen-2020-046235] [PMID: ] - DOI - PMC - PubMed
Madathil 2021b {published data only}
-
- Madathil S, Thomas D, Chandra P, Agarwal R, Sankar MJ, Thukral A, et al. 'NOPAIN-ROP' trial: intravenous fentanyl and intravenous ketamine for pain relief during laser photocoagulation for retinopathy of prematurity (ROP) in preterm infants: a randomised trial. BMJ Open 2021;11(9):e046235. [DOI: 10.1136/bmjopen-2020-046235] [PMID: ] - DOI - PMC - PubMed
Manjunatha 2009 {published data only}
Pokela 1994 {published data only}
-
- Pokela ML. Pain relief can reduce hypoxemia in distressed neonates during routine treatment procedures. Pediatrics 1994;93(3):379-83. [PMID: ] - PubMed
Sethi 2020 {published data only}
-
- CTRI/2017/07/008977. A clinical study to compare the effect of fentanyl and oral sucrose in providing optimal pain relief in preterm babies during laser therapy for retinopathy of prematurity. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2017/07/008977 (first received 5 July 2017). [CENTRAL: CN-01887047]
-
- Sethi A, Sankar MJ, Kulkarni S, Thukral A, Chandra P, Agarwal R. Low dose fentanyl infusion versus 24% oral sucrose for pain management during laser treatment for retinopathy of prematurity-an open label randomized clinical trial. European Journal of Pediatrics 2020;179(2):285-92. [DOI: 10.1007/s00431-019-03514-5] [PMID: ] - DOI - PubMed
Sindhur 2020 {published data only}
-
- Sindhur M, Balasubramanian H, Srinivasan L, Kabra NS, Agashe P, Doshi A. Intranasal fentanyl for pain management during screening for retinopathy of prematurity in preterm infants: a randomized controlled trial. Journal of Perinatology 2020;40(6):881-7. [DOI: 10.1038/s41372-020-0608-2] [PMID: ] - DOI - PubMed
Taddio 2006 {published data only}
-
- NCT00213200. Intravenous and topical analgesics for procedural pain in neonates. clinicaltrials.gov/show/NCT00213200 (first received 21 September 2005). [CENTRAL: CN-02013389]
-
- Taddio A, Lee C, Yip A, Parvez B, McNamara PJ, Shah V. Intravenous morphine and topical tetracaine for treatment of pain in preterm neonates undergoing central line placement. JAMA 2006;295(7):793‐800TN Erratum in JAMA. 2006 Apr 5;295(13):1518. - PubMed
References to studies excluded from this review
Axelin 2009 {published data only}
-
- Axelin A, Salantera S, Kirjavainen J, Lehtonen L. Oral glucose and parental holding superior to opioid in pain management in preterm infants. In: American Pediatric Society/Society for Pediatric Research Abstract. 2008.
Bell 2019 {published data only}
-
- Bell JL, Hardy P, Stallard N, Slater R, Hartley C, Adams E, et al. Setting up a stopping boundary for safety in a phase II trial: the Poppi trial. In: paediatrics.ox.ac.uk/publications/1070177. Vol. 20. 2019.
Campbell‐Yeo 2018 {published data only}
Chambers 2002 {published data only}
Moustogiannis 1996 {published data only}
NCT00571636 {published data only}
-
- NCT00571636. Continuous infusion of fentanyl in preterm on MV. clinicaltrials.gov/show/NCT00571636 (first received 12 December 2007). [CENTRAL: CN-02018526]
NCT03897452 {published data only}
-
- NCT03897452. NeoFent-I study; fentanyl treatment in newborn infants; a pharmacokinetic, pharmacodynamic and pharmacogenetic study. clinicaltrials.gov/ct2/show/NCT03897452 (first received 1 April 2019).
Rosen 2000 {published data only}
Shin 2013 {published data only}
-
- Shin S, Lee J, Choi K, Kim Y, Kim E. Comparative study of two dose of remifentanil for procedural pain in ventilated preterm infants; a randomized controlled study. In: Pediatric Academic Societies Annual Meeting. 2013. - PubMed
Shin 2014 {published data only}
Soffer 2019 {published data only}
Valkenburg 2015 {published data only}
References to studies awaiting assessment
ACTRN12612000385842 {published data only}
-
- ACTRN12612000385842. A randomised double-blind placebo-controlled trial of the efficacy of remifentanil for procedural pain in neonates. impact.psanz.com.au/clinical-trials/clinical-trials-2/?Sort=Topic&Di... (first received 4 April 2012). [CENTRAL: CN-01882516]
Gadzinowski 2000 {published data only}
-
- Gadzinowski J, Vidyasagar D, Bhat R, Rozycka J. Effect of short-term morphine infusion on premature infant pain profile (PIPP) and hemodynamics. In: The Abstracts of the XVII European Congress of Perinatal Medicine 2000. 2000.
Li 1997 {published data only}
-
- Li HK, Norcross-Nechay K, Marquez BA, Dejean BJ, Khorrami AM, Richardson J. Pain and stimulation in laser treatment for retinopathy of prematurity. In: IOVS 1997;38:ARVO Abstract 4515. 1997.
References to ongoing studies
CTRI/2017/12/011035 {published data only}
-
- CTRI/2017/12/011035. Single dose intranasal fentanyl for the reduction of pain associated with laser therapy for retinopathy of prematurity: a randomized controlled trial. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2017/12/011035 (first received 29 December 2017). [CENTRAL: CN-01900138]
CTRI/2018/04/012926 {published data only}
-
- CTRI/2018/04/012926. Oral morphine for analgesia in neonates undergoing ROP screening. trialsearch.who.int/?TrialID=CTRI/2018/04/012926 (first received 2 April 2018). [CENTRAL: CN-01904425]
CTRI/2020/08/027144 {published data only}
-
- CTRI/2020/08/027144. Analgesia during less invasive surfactant administration (LISA): a randomized controlled trial [Pain reducing drug administration during surfactant therapy by less invasive technique. Surfactant are alveolar surface reducing agents, used in baby having lower pool surfactant i.e. respiratory distress syndrome]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2020/08/027144 (first received 14 August 2020). [CENTRAL: CN-02185616]
NCT02125201 {published data only}
-
- NCT02125201. Effectiveness of intranasal versus intravenous fentanyl in preterm and term newborns for pain prevention. clinicaltrials.gov/ct2/show/NCT02125201 (first received 29 April 2014). [CENTRAL: CN-01545341]
NCT03718507 {published data only}
-
- NCT03718507. Study on the effects of different premedication for LISA on stress and cerebral tissue oxygenation in preterm infants (SAFE LISA). clinicaltrials.gov/ct2/show/NCT03718507 (first received 24 October 2018). [CENTRAL: CN-01664417]
NCT03735563 {published data only}
-
- NCT03735563. Premedication for less invasive surfactant administration. clinicaltrials.gov/show/NCT03735563 (first received 8 November 2018). [CENTRAL: CN-01918347]
NCT04073173 {published data only}
-
- NCT04073173. Stress assessment with and without analgesia during surfactant therapy in preterm infants. clinicaltrials.gov/show/NCT04073173 (first received 29 August 2019). [CENTRAL: CN-01983867]
Additional references
AAP 2016
Allegaert 2020
Anand 1999
-
- Anand KJ, Barton BA, McIntosh N, Lagercrantz H, Pelausa E, Young TE, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Archives of Pediatrics and Adolescent Medicine 1999;153(4):331-8. [DOI: 10.1001/archpedi.153.4.331] [PMID: ] - DOI - PubMed
Anand 2005
Anand 2007
Andersen 2010
-
- Andersen RD, Meberg A, Wallin L, Jylli L. Clinical staff perceptions of the treatment of procedural pain in neonates: exploring a change process. International Journal of Medical and Biological Frontiers 2010;16(5/6):401-14. [ISSN: 1081-3829]
Antmen 2005
-
- Antmen B, Șașmaz I, Birbiçer H, Özbek H, Burgut R, Ișik G, et al. Safe and effective sedation and analgesia for bone marrow aspiration procedures in children with alfentanil, remifentanil and combinations with midazolam. Paediatric Anaesthesia 2005;15(3):214-9. [DOI: 10.1111/j.1460-9592.2004.01411.x] [PMID: ] - DOI - PubMed
Ayed 2017
-
- Ayed M, Shah VS, Taddio A. Premedication for non-urgent endotracheal intubation for preventing pain in neonates. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD012562. [DOI: 10.1002/14651858.CD012562] - DOI
Balan 2018
Bali 2015
Bayley 1993
-
- Bayley N. Bayley Scales of Infant Development–II. San Antonio, Texas: Psychological Corporation, 1993.
Bayley 2005
-
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio, TX: Harcourt Assessment, 2005.
Bellieni 2018
Bellieni 2005
Bellù 2021
Borenstein 2013
Bruschettini 2022
-
- Bruschettini M, Olsson E, Persad E, Garratt A, Roger S. Clinical rating scales for assessing pain in newborn infants. Cochrane Database of Systematic Reviews 2022, Issue 2. Art. No: MR000064. [DOI: 10.1002/14651858.MR000064] - DOI
Butt 2013
Bäcke 2022
-
- Bäcke P, Bruschettini M, Sibrecht G, Thernström Blomqvist Y, Olsson E. Pharmacological interventions for pain and sedation management in newborn infants undergoing therapeutic hypothermia. Cochrane Database of Systematic Reviews 2022, Issue 11. Art. No: CD015023. [DOI: 10.1002/14651858.CD015023] - DOI - PMC - PubMed
CADTH 2016
-
- Canadian Agency for Drugs and Technologies in Health. Strings attached: CADTH database search filters. www.cadth.ca/strings-attached-cadths-database-search-filters (accessed 01 October 2021).
Carbajal 2005
Carbajal 2008
Carbajal 2015
-
- Carbajal R, Eriksson M, Courtois E, Boyle E, Avila-Alvarez A, Andersen RD, et al, EUROPAIN Survey Working Group. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Respiratory Medicine 2015;3(10):796-812 [Erratum in: Lancet Respir Med. 2015 Dec;3(12):e44. doi: 10.1016/S2213-2600(15)00482-8. Epub 2015 Nov 30]. [DOI: 10.1016/S2213-2600(15)00331-8] [PMID: ] - DOI - PubMed
Carbajal 1997
Cerchione 2020
-
- Cerchione C, Martinelli G, Picardi M, Pugliese N, Nappi D, Casoria A, et al. Combined oral fentanyl citrate and midazolam as premedication for bone marrow aspiration and biopsy in patients with hematological malignancies: a randomized, controlled and patient-blinded clinical trial. Journal of Clinical Medicine 2020;9(2):395. [DOI: 10.3390/jcm9020395] [PMID: ] - DOI - PMC - PubMed
Cignacco 2004
Cochrane EPOC Group 2017
-
- Cochrane Effective Practice, Organisation of Care (EPOC). Data extraction and management. EPOC resources for review authors. Available from: epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 01 October 2021).
Courtois 2016
-
- Courtois E, Droutman S, Magny JF, Merchaoui Z, Durrmeyer X, Roussel C, et al. Epidemiology and neonatal pain management of heelsticks in intensive care units: EPIPPAIN 2, a prospective observational study. International Journal of Nursing Studies 2016;59:79-88. [DOI: 10.1016/j.ijnurstu.2016.03.014] [PMID: ] - DOI - PubMed
Cruz 2016
Deeks 2020
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Deeks 2022
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Dotta 2011
Duffett 2012
Gibbins 2014
Giordano 2019
Gitto 2012
-
- Gitto E, Pellegrino S, Manfrida M, Aversa S, Trimarchi G, Barberi I, et al. Stress response and procedural pain in the preterm newborn: the role of pharmacological and non-pharmacological treatments. European Journal of Pediatrics 2012;171(6):927-33. [DOI: 10.1007/s00431-011-1655-7] [PMID: ] - DOI - PubMed
Gomes Neto 2020
-
- Gomes Neto M, da Silva Lopes IA, Morais AC, Oliveira LS, Saquetto MB. The effect of facilitated tucking position during painful procedure in pain management of preterm infants in neonatal intensive care unit: a systematic review and meta-analysis. European Journal of Pediatrics 2020;179(5):699-709. [DOI: 10.1007/s00431-020-03640-5] [PMID: ] - DOI - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 11 January 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Griffiths 1954
-
- Griffiths R. The abilities of babies: a study of mental measurement. London: University of London Press, 1954.
Griffiths 1970
-
- Griffiths R. The abilities of young children: a comprehensive system of mental measurement for the first eight years. London: Child Development Research Center, 1970.
Guinsburg 1998
Hall 2014
Hartley 2018
-
- Hartley C, Moultrie F, Hoskin A, Green G, Monk V, Bell JL, et al. Analgesic efficacy and safety of morphine in the Procedural Pain in Premature Infants (Poppi) study: randomised placebo-controlled trial. The Lancet 2018;392(10164):2595-605. [DOI: 10.1016/S0140-6736(18)31813-0] [PMID: ] - DOI - PMC - PubMed
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2021
-
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Holsti 2008
Hummel 2008
-
- Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. Journal of Perinatology: Official Journal of the California Perinatal Association 2008;28(1):55-60. [DOI: 10.1038/sj.jp.7211861] [PMID: ] - DOI - PubMed
Hällström 2003
Jacobs 2013
Jiang 2012
-
- Jiang H, Cheng R, Kan Q, Shen X, Li F, Fu, C et al. Clinical evaluation of the effects of morphine in mechanical ventilation of neonates. Zhonghua Er Ke Za Zhi 2012;50:350-5. [PMID: ] - PubMed
Johnston 2013
Kassab 2019
-
- Kassab M, Alhassan A, Alzoubi K, Khader Y. Number and frequency of routinely applied painful procedures in university neonatal intensive care unit. Clinical Nursing Research 2019;28(4):488-501. [DOI: ] [PMID: ] - PubMed
Khanafer‐Larocque 2019
-
- Khanafer-Larocque I, Soraisham A, Stritzke A, Al Awad E, Thomas S, Murthy P, et al. Intraventricular hemorrhage: risk factors and association with patent ductus arteriosus treatment in extremely preterm neonates. Frontiers in Pediatrics 2019;22:408. [DOI: 10.3389/fped.2019.00408] [PMID: ] - DOI - PMC - PubMed
Kinoshita 2020
Kinoshita 2021
Kinoshita 2023
Krzyżaniak 2016
Ku 2019
Lawrence 1993
-
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12(6):59-66. [PMID: ] - PubMed
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: Searching for and selecting studies. Section 4.S2 Supplementary material: Appendix of resources. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). In: Higgins JP Thomas J Chandler J Cumpston M Li T Page MJ Welch VA, editors(s). Available from training.cochrane.org/handbook/archive/v6.2. Cochrane, 2021. [https://training.cochrane.org/handbook/current/chapter-04-appendix-resou...]
Marshall 2018
Maxwell 2019
McGowan 2016a
-
- McGowan J, Sampson M, Salzwedel D, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline Explanation and Elaboration (PRESS E&E). Ottawa: CADTH, 2016. [URL: https://www.cadth.ca/sites/default/files/pdf/CP0015_PRESS_Update_Report_...] - PubMed
McGowan 2016b
McKechnie 2008
McPherson 2015
Mehler 2013
Moher 2009
Monk 2019
-
- Monk V, Moultrie F, Hartley C, Hoskin A, Green G, Bell JL, et al. Oral morphine analgesia for preventing pain during invasive procedures in non-ventilated premature infants in hospital: the Poppi RCT. Efficacy and Mechanism Evaluation, No. 6.9. Southampton (UK): NIHR Journals Library 2019. [DOI: 10.3310/eme06090] - DOI - PubMed
Nafziger 2018
Noel‐Storr 2020
-
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomised controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [PMID: ] - DOI - PubMed
Noel‐Storr 2021
-
- Noel-Storr AH, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomised trials. Journal of Clinical Epidemiology 2021;4356(21):00008-1. [DOI: 10.1016/j.jclinepi.2021.01.006] [PMID: ] - DOI - PubMed
Norman 2019
Ohlsson 2020
Olsson 2021
Orsini 1996
Osborn 2021
Papile 1978
Pirlotte 2019
Prakeshkumar 2011
Ranger 2013
Ranger 2014
RCT‐Filter EMBASE
-
- Cochrane. How CENTRAL is created: Identifying RCTs for inclusion in CENTRAL. Available from: https://www.cochranelibrary.com/central/central-creation (accessed 01 October 2021). [cochranelibrary.com/central/central-creation]
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Riskin 2015
-
- Riskin A, Shoris I, Kidalov G, Bader D, Borenstein-levin L, Kugelman A. Opiates use in very low birth weight infants—call for caution. Harefuah 2015;154:757-60. [PMID: ] - PubMed
Romantsik 2020
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Scott 1999
Shah 2012
Simons 2003
Squillaro 2019
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Stevens 1996
Thigpen 2019
Thomas 2020
-
- Thomas J, McDonald S, Noel-Storr AH, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduces workload with minimal risk of missing studies: development and evaluation of an RCT classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2020;S0895-4356(20):31172-0. [DOI: 10.1016/j.jclinepi.2020.11.003vcvc] [PMID: ] - DOI - PMC - PubMed
Tibboel 2005
van den 2016
Walker 2019
Walsh 1986
Williams 2020
Zvizdic 2019
Zwicker 2016
References to other published versions of this review
Kinoshita 2021
-
- Kinoshita M, Olsson E, Borys F, Bruschettini M. Opioids for procedural pain in neonates. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD015056. [DOI: 10.1002/14651858.CD015056] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

