Hematopoietic stem-cell gene therapy is associated with restored white matter microvascular function in cerebral adrenoleukodystrophy
- PMID: 37019892
- PMCID: PMC10076264
- DOI: 10.1038/s41467-023-37262-w
Hematopoietic stem-cell gene therapy is associated with restored white matter microvascular function in cerebral adrenoleukodystrophy
Abstract
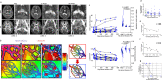
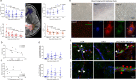
Blood-brain barrier disruption marks the onset of cerebral adrenoleukodystrophy (CALD), a devastating cerebral demyelinating disease caused by loss of ABCD1 gene function. The underlying mechanism are not well understood, but evidence suggests that microvascular dysfunction is involved. We analyzed cerebral perfusion imaging in boys with CALD treated with autologous hematopoietic stem-cells transduced with the Lenti-D lentiviral vector that contains ABCD1 cDNA as part of a single group, open-label phase 2-3 safety and efficacy study (NCT01896102) and patients treated with allogeneic hematopoietic stem cell transplantation. We found widespread and sustained normalization of white matter permeability and microvascular flow. We demonstrate that ABCD1 functional bone marrow-derived cells can engraft in the cerebral vascular and perivascular space. Inverse correlation between gene dosage and lesion growth suggests that corrected cells contribute long-term to remodeling of brain microvascular function. Further studies are needed to explore the longevity of these effects.
© 2023. The Author(s).
Conflict of interest statement
This work was funded primarily by NINDS K08 NS094683-01 and R01NS117575 with a partial contribution from Bluebird Bio. D.J.L. is a consultant for Bluebird Bio. D.W. has received research funding for research in hemoglobinopathies and licensed certain IP relevant to hemoglobinopathies to Bluebird Bio. He has received payments from Bluebird Bio in past through BCH institutional licensing agreement and has the potential for future royalty/milestone income. P.L.M. is the co-I of Bluebird Bio clinical trials. She is a consultant to bluebird bio. F.S.E. is the co-PI of Bluebird Bio clinical trials. M.B.H and K.M. are co-applicants on a patent application based on the presented techniques in this manuscript (PCT/DK2014/050296) and are shareholders in Cercare Medical ApS. A.L.; S.L.S.; M.C.; X.D.; C.D.; S.M.C.; V.K.; C.A.L.; D.R.; J.K.-C.; K.E., and P.C. do not have any disclosures to report directly relating to this study.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

